After reading this chapter you will be able to: Respiratory failure is a clinical problem that all respiratory care practitioners must be skilled at identifying, assessing, and treating. A 1994 study of more than 1400 patients concluded that 44% of patients diagnosed with acute respiratory failure requiring intensive care unit (ICU) admission died in the hospital.1 A review of hospital discharge records from 2005 in six states in the United States showed only marginal improvement, with hospital mortality of 34.5%.2 The need for oxygen (O2) delivery, mechanical ventilation, and other modalities makes the respiratory therapist (RT) indispensable in the treatment of this life-threatening condition. Respiratory failure is the “inability to maintain either the normal delivery of O2 to the tissues or the normal removal of carbon dioxide (CO2) from the tissues”3 and often results from an imbalance between respiratory workload and ventilatory strength or endurance. Criteria for respiratory failure based on arterial blood gases (ABGs) were established by Campbell4 and generally define failure as arterial partial pressure of oxygen (PaO2) less than 60 mm Hg or alveolar partial pressure of carbon dioxide (PaCO2) greater than 50 mm Hg (or both) in otherwise healthy individuals breathing room air at sea level. Respiratory failure can be an acute or a chronic process. Additionally and classically, it has also been separated into two other categories to reflect the type of physiologic impairment. Hypoxemic (type I) respiratory failure occurs when the primary problem is inadequate O2 delivery. Hypercapnic (type II) respiratory failure describes “bellows failure” of the lungs resulting in elevated CO2 levels. Hypercapnic respiratory failure is also known as ventilatory failure. Patients with baseline acid-base derangement (e.g., chronic obstructive pulmonary disease [COPD], restrictive lung disease) may be chronically hypercapnic and in chronic ventilatory failure based on the guidelines. These individuals develop acute failure when their chronic state deteriorates significantly; this is sometimes referred to as acute ventilatory failure superimposed on chronic ventilatory failure. The primary causes of hypoxemia are the following: These entities are briefly discussed here and are discussed in more detail in Chapters 10 and 11. There are regions in healthy lungs where ventilation and perfusion are not evenly matched, so it seems logical that this is the most common cause of hypoxemia. RTs are familiar with this concept through the work of West,5 which described a high Pathologic Because patients present with hypoxemia, the initial goal is always to treat the low PaO2 or SpO2 (arterial O2 saturation by pulse oximeter). Shunt is an extreme version of The clinical presentation of shunt is very similar in many ways to the presentation of Diffusion refers to movement of gas across the alveolar-capillary membrane secondary to a pressure gradient. Although diffusion is rarely a cause of significant hypoxemia at rest, its effects become more pronounced with exercise, which limits the time for gas exchange. This time limitation is normally not a problem, unless diffusion impairment is present, in which case hypoxemia results. Diffusion impairment in interstitial lung disease may contribute 20% to 30% of the widening in the alveolar-arterial O2 gradient during exercise.7 Diffusion impairment most commonly manifests in patients with interstitial lung disease (e.g., pulmonary fibrosis, asbestosis, sarcoidosis) in which the thickening and scarring of the interstitium undermine normal gas exchange. Emphysema, with its inherent alveolar destruction, also has subnormal transfer of O2 and CO2 between the alveolus and the capillary. The reduced ventilation in both diseases implies that Perfusion/diffusion impairment is a rare cause of hypoxemia found in individuals with liver disease complicated by hepatopulmonary syndrome.6 In this condition, right-to-left intracardiac shunt combines with dilated pulmonary capillaries resulting in impaired gas exchange. Specifically, the normal alveolar partial pressures of O2 may be insufficient to drive the O2 molecules to the center of the dilated pulmonary vasculature. Cirrhosis is the most common liver disease, and portal hypertension is usually present. Although shunt is a component of the syndrome, significant supplemental O2 can overcome the gas transfer reduction owing to the dilated vessels, so this is commonly called a perfusion/diffusion defect. Also clinically uncommon, hypoxemia may develop when the inspired O2 is less than body requirements. The most common situation is at high altitude, where barometric pressure decreases, which results in a decrease in the partial pressure of inspired O2. Although airlines account for this decrease in barometric pressure by pressurizing their cabins, travelers with chronic hypoxemia may still need supplemental O2.8 Similarly, mountain climbers sometimes require O2 masks. Cases of patient-O2 disconnects and delivery of an incorrect gas source, which, it is hoped, occur rarely, are also included in this category. Inspired O2 less than 21% can be used diagnostically and therapeutically. The Hypoxia Altitude Simulation Test seeks to replicate inspired partial pressure of O2 (PiO2) during air travel by asking the potential traveler to inhale a hypoxic mixture. Inhaling at FiO2 of 15% replicates the PiO2 found at an altitude of 8000 feet (108 mm Hg). For a lower altitude of 5400 feet, an equivalent FiO2 of 17% can be calculated.8 Infants with certain cyanotic congenital heart defects (e.g., hypoplastic left ventricle) may benefit from FiO2 below room air level. In the preoperative state, low FiO2 helps to prevent pulmonary dilation and the excessive pulmonary blood flow, which could flood the lungs. A decrease in mixed venous O2 increases the gradient by which O2 needs to be stepped up as it passes through the lungs and can contribute to the development of hypoxemia. Congestive heart failure with low cardiac output is the most common cause of low mixed venous O2, owing to increased peripheral extraction of O2. Other causes include low hemoglobin concentration and increased O2 consumption. A low mixed venous O2 may have a significant effect on the ultimate arterial O2 tension in the presence of lung disease. There may be other, more important coexisting determinants of hypoxemia, such as It is important to recognize the physiologic basis of each of the three main causes of hypoxemic respiratory failure (hypoventilation, TABLE 41-1 Differentiating the Cause of Hypoxemia A
Respiratory Failure and the Need for Ventilatory Support
 Define acute respiratory failure.
Define acute respiratory failure.
 Differentiate between hypoxemic respiratory failure (type I) and hypercapnic respiratory failure (type II).
Differentiate between hypoxemic respiratory failure (type I) and hypercapnic respiratory failure (type II).
 Discuss the causes of acute respiratory failure.
Discuss the causes of acute respiratory failure.
 Discuss the differences between chronic respiratory failure and acute-on-chronic respiratory failure.
Discuss the differences between chronic respiratory failure and acute-on-chronic respiratory failure.
 Identify the complications of respiratory failure.
Identify the complications of respiratory failure.
 Discuss the indication for ventilatory support.
Discuss the indication for ventilatory support.
 Discuss general management principles of hypoxemic and hypercapnic respiratory failure.
Discuss general management principles of hypoxemic and hypercapnic respiratory failure.
Hypoxemic Respiratory Failure (Type I)
Ventilation/Perfusion Mismatch
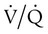 ratio at the apex of the lungs and a low ratio at the bases. This concept can be oversimplified and stated as there being more air than blood at the apices and more blood than air at the bases.
ratio at the apex of the lungs and a low ratio at the bases. This concept can be oversimplified and stated as there being more air than blood at the apices and more blood than air at the bases.
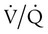 mismatch occurs when disease disrupts this balance, and hypoxemia results (Figure 41-1, A). Most commonly, areas of low
mismatch occurs when disease disrupts this balance, and hypoxemia results (Figure 41-1, A). Most commonly, areas of low 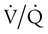 ratio are seen in which ventilation is compromised despite adequate blood flow. Obstructive lung diseases are frequent causes. The bronchospasm, mucous plugging, inflammation, and premature airway closure that signal asthmatic or emphysematous exacerbations worsen ventilation and create
ratio are seen in which ventilation is compromised despite adequate blood flow. Obstructive lung diseases are frequent causes. The bronchospasm, mucous plugging, inflammation, and premature airway closure that signal asthmatic or emphysematous exacerbations worsen ventilation and create 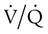 mismatch. Infection, heart failure, and inhalation injury may lead to partially collapsed or fluid-filled alveoli, also resulting in decreased ventilation and reduced blood O2 levels.
mismatch. Infection, heart failure, and inhalation injury may lead to partially collapsed or fluid-filled alveoli, also resulting in decreased ventilation and reduced blood O2 levels.
Clinical Presentation
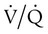 mismatch responds to supplemental O2 (see Figure 41-1, B). Hypoxemia commonly manifests with dyspnea, tachycardia, and tachypnea, but these are very nonspecific findings. However, patient observation is extremely valuable. The use of accessory muscles of respiration (scalene, pectoralis major, and sternomastoid) is an important sign that normal diaphragmatic inspiration is inadequate. In an elderly, cachectic, or barrel-chested individual who is leaning forward on his or her arms, COPD is the likely diagnosis. Nasal flaring may be present. Lower extremity edema is more indicative of cardiac failure as the cause of hypoxemia. Cyanosis may be peripheral and primarily due to decreased blood flow. Central cyanosis, seen most easily as a bluish tint around the lips, occurs when greater than 5 g/dl of unsaturated hemoglobin is present. This finding is more common in patients with polycythemia but may be subject to wide observer variability. More severe hypoxemia can lead to significant central nervous system (CNS) dysfunction, ranging from irritability to confusion to coma.
mismatch responds to supplemental O2 (see Figure 41-1, B). Hypoxemia commonly manifests with dyspnea, tachycardia, and tachypnea, but these are very nonspecific findings. However, patient observation is extremely valuable. The use of accessory muscles of respiration (scalene, pectoralis major, and sternomastoid) is an important sign that normal diaphragmatic inspiration is inadequate. In an elderly, cachectic, or barrel-chested individual who is leaning forward on his or her arms, COPD is the likely diagnosis. Nasal flaring may be present. Lower extremity edema is more indicative of cardiac failure as the cause of hypoxemia. Cyanosis may be peripheral and primarily due to decreased blood flow. Central cyanosis, seen most easily as a bluish tint around the lips, occurs when greater than 5 g/dl of unsaturated hemoglobin is present. This finding is more common in patients with polycythemia but may be subject to wide observer variability. More severe hypoxemia can lead to significant central nervous system (CNS) dysfunction, ranging from irritability to confusion to coma.
Shunt
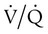 mismatch in which there is no ventilation to match perfusion (
mismatch in which there is no ventilation to match perfusion (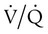 = 0). About 2% to 3% of the blood supply is shunted via the bronchial and thebesian veins that feed the lungs and heart; this is normal anatomic shunt. Pathologic anatomic shunt occurs as a result of right-to-left blood flow through cardiac openings (e.g., atrial or ventricular septal defects) or in pulmonary arteriovenous malformations. Physiologic shunt leads to hypoxemia when alveoli collapse or are filled with fluid or exudate. Common etiologies of physiologic shunting include atelectasis, pulmonary edema, and pneumonia. In contrast to
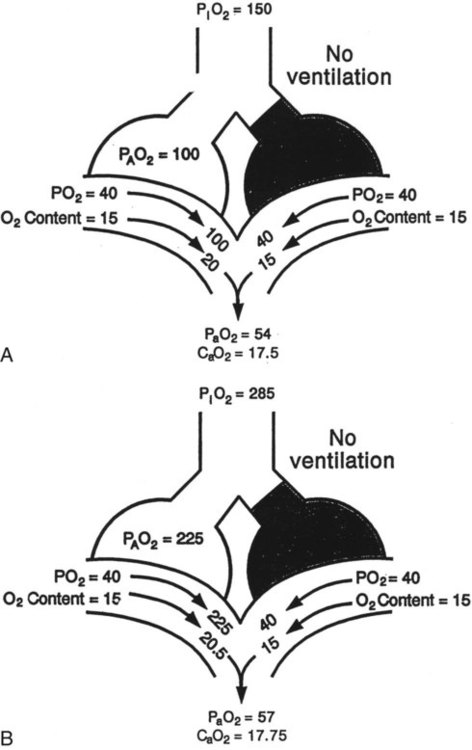
= 0). About 2% to 3% of the blood supply is shunted via the bronchial and thebesian veins that feed the lungs and heart; this is normal anatomic shunt. Pathologic anatomic shunt occurs as a result of right-to-left blood flow through cardiac openings (e.g., atrial or ventricular septal defects) or in pulmonary arteriovenous malformations. Physiologic shunt leads to hypoxemia when alveoli collapse or are filled with fluid or exudate. Common etiologies of physiologic shunting include atelectasis, pulmonary edema, and pneumonia. In contrast to 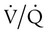 mismatch, shunt does not respond to supplemental O2 because the gas exchange unit (the alveolus) is not open (Figure 41-2, A).
mismatch, shunt does not respond to supplemental O2 because the gas exchange unit (the alveolus) is not open (Figure 41-2, A).
 as illustrated in Figure 41-1. (Modified from Pierson DJ, Kacmarek RM: Foundations of respiratory care, New York, 1992, Churchill Livingstone.)
as illustrated in Figure 41-1. (Modified from Pierson DJ, Kacmarek RM: Foundations of respiratory care, New York, 1992, Churchill Livingstone.)
Clinical Presentation
 mismatch. Patient observations are similar, although chest excursion occasionally may be asymmetric in shunt. Bilateral or unilateral crackles are common owing to the alveolar filling process. Unilateral absence of breath sounds may indicate significant collapse, mass, or effusion; these conditions require treatment before oxygenation can improve. Shunt usually manifests with a “white” chest radiograph. The most advanced example of this is the diffuse, bilateral haziness in acute respiratory distress syndrome (ARDS). Shunting can be diagnosed by using 100% O2 breathing techniques, contrast-enhanced echocardiography, macroaggregated albumin scanning, or pulmonary angiography.6 Shunt is differentiated from
mismatch. Patient observations are similar, although chest excursion occasionally may be asymmetric in shunt. Bilateral or unilateral crackles are common owing to the alveolar filling process. Unilateral absence of breath sounds may indicate significant collapse, mass, or effusion; these conditions require treatment before oxygenation can improve. Shunt usually manifests with a “white” chest radiograph. The most advanced example of this is the diffuse, bilateral haziness in acute respiratory distress syndrome (ARDS). Shunting can be diagnosed by using 100% O2 breathing techniques, contrast-enhanced echocardiography, macroaggregated albumin scanning, or pulmonary angiography.6 Shunt is differentiated from  mismatch by the lack of increase in PO2 as fractional inspired oxygen (FiO2) is increased (see Figure 41-2, B).
mismatch by the lack of increase in PO2 as fractional inspired oxygen (FiO2) is increased (see Figure 41-2, B).
Diffusion Impairment
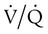 mismatch also plays a role in the resulting hypoxemia.
mismatch also plays a role in the resulting hypoxemia.
Perfusion/Diffusion Impairment
Decreased Inspired Oxygen
Venous Admixture
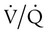 mismatch and shunting.3
mismatch and shunting.3
Differentiating the Causes of Acute Hypoxemic Respiratory Failure
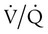 mismatch, and shunt). Hypoventilation differs from the other causes in manifesting with a normal alveolar-to-arterial PO2 difference [P(A − a)O2] indicating normal lung parenchyma (Table 41-1). A clinical determination of this difference is made by subtracting PaO2 from PAO2 (partial pressure of alveolar O2) derived from the alveolar air equation:
mismatch, and shunt). Hypoventilation differs from the other causes in manifesting with a normal alveolar-to-arterial PO2 difference [P(A − a)O2] indicating normal lung parenchyma (Table 41-1). A clinical determination of this difference is made by subtracting PaO2 from PAO2 (partial pressure of alveolar O2) derived from the alveolar air equation:
Cause
P(a − a)O2
Response to Increased FiO2
Hypoventilation
Normal
Marked
Shunt
Increased
Minimal
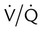 mismatch
mismatch
Increased
Marked
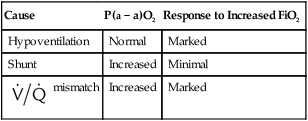
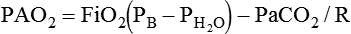
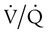 mismatch and shunt both result in elevated P(A − a)O2 levels, indicating that the resultant hypoxemia is due to an abnormality of lung tissue, requiring treatment to address that abnormality. When the RT encounters an increased P(A − a)O2, a
mismatch and shunt both result in elevated P(A − a)O2 levels, indicating that the resultant hypoxemia is due to an abnormality of lung tissue, requiring treatment to address that abnormality. When the RT encounters an increased P(A − a)O2, a 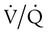 mismatch and shunt can be differentiated by means of O2 administration (see Figures 41-1 and 41-2). A significant response to applying even small amounts of O2 identifies
mismatch and shunt can be differentiated by means of O2 administration (see Figures 41-1 and 41-2). A significant response to applying even small amounts of O2 identifies 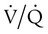 mismatch as the cause of hypoxemia because altered P(A − a)O2 has not been totally obliterated. True shunt shows little or no improvement in oxygenation even with 100% FiO2 (see Table 41-1). As a result, treatment of intrapulmonary shunt must be directed toward opening collapsed alveoli or clearing fluid or exudative material before O2 can be beneficial at below toxic levels. Testing to rule out anatomic shunt should be done in the right clinical setting (e.g., clear or black parenchyma on the chest radiograph).
mismatch as the cause of hypoxemia because altered P(A − a)O2 has not been totally obliterated. True shunt shows little or no improvement in oxygenation even with 100% FiO2 (see Table 41-1). As a result, treatment of intrapulmonary shunt must be directed toward opening collapsed alveoli or clearing fluid or exudative material before O2 can be beneficial at below toxic levels. Testing to rule out anatomic shunt should be done in the right clinical setting (e.g., clear or black parenchyma on the chest radiograph).


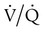 ) mismatch
) mismatch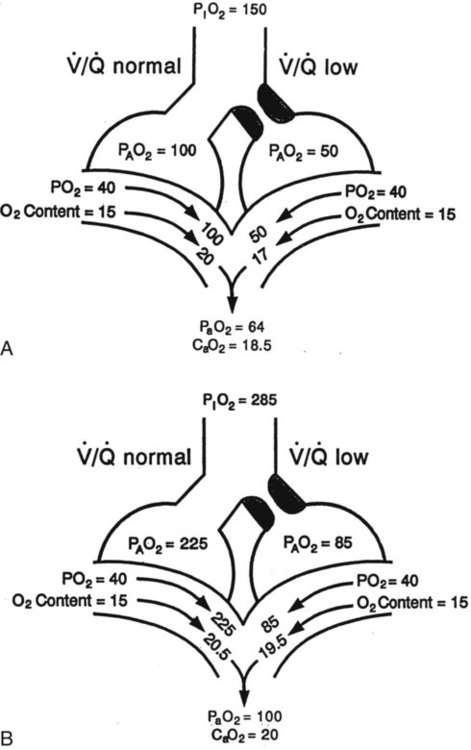
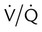 mismatch showing the effect of supplemental O2.
mismatch showing the effect of supplemental O2. 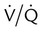 is normal on the left side of each idealized lung unit and low on the right. Only O2 exchange is shown, and P(A − a)O2 is assumed to be zero. A, With room air, not enough O2 reaches the poorly ventilated alveolus to saturate its capillary blood fully. B, With 40% O2, PaO2 in this alveolus is increased enough to make capillary PO2 nearly normal. PaO2 in the mixed blood from the two capillaries is determined by the average of the O2 contents of the two streams of blood, not by the PaO2 values.
is normal on the left side of each idealized lung unit and low on the right. Only O2 exchange is shown, and P(A − a)O2 is assumed to be zero. A, With room air, not enough O2 reaches the poorly ventilated alveolus to saturate its capillary blood fully. B, With 40% O2, PaO2 in this alveolus is increased enough to make capillary PO2 nearly normal. PaO2 in the mixed blood from the two capillaries is determined by the average of the O2 contents of the two streams of blood, not by the PaO2 values. 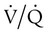 mismatch. Unilateral crackles generally indicate an alveolar filling process (mass, infection, fluid).
mismatch. Unilateral crackles generally indicate an alveolar filling process (mass, infection, fluid).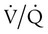 mismatch can manifest as a “black” radiograph, with large or hyperinflated lungs as in the case of obstructive disease. A “white” chest radiograph is evident when alveoli are partially occluded. The “blackness” or “whiteness” of the lung fields on the plain chest radiograph has important diagnostic value in assessing a patient with acute respiratory failure.
mismatch can manifest as a “black” radiograph, with large or hyperinflated lungs as in the case of obstructive disease. A “white” chest radiograph is evident when alveoli are partially occluded. The “blackness” or “whiteness” of the lung fields on the plain chest radiograph has important diagnostic value in assessing a patient with acute respiratory failure.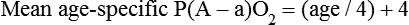
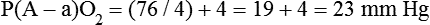
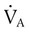 ) are inversely related, meaning that alveolar and arterial PCO2 levels are doubled when alveolar ventilation is halved. This is illustrated by the relationship:
) are inversely related, meaning that alveolar and arterial PCO2 levels are doubled when alveolar ventilation is halved. This is illustrated by the relationship: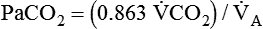
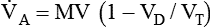
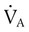 is alveolar ventilation (L/min), MV is minute ventilation, VD/VT is dead space-to-tidal volume ratio, and
is alveolar ventilation (L/min), MV is minute ventilation, VD/VT is dead space-to-tidal volume ratio, and 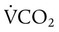 is CO2 production (ml/min).
is CO2 production (ml/min).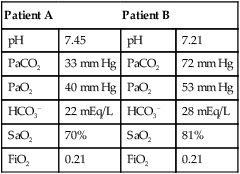
 mismatch as the cause, whereas shunt would be implicated if PaO2 did not respond to the increase in delivered O2. In the latter condition, some form of PEEP would be necessary to improve gas exchange by improving functional residual capacity. Patient B has hypercapnic respiratory failure (ventilatory failure) with hypoxemia, but with P(A − a)O2 of 7 mm Hg, which is within the normal range. A pure ventilatory defect is the cause of hypoxemia, and administration of 100% FiO2
mismatch as the cause, whereas shunt would be implicated if PaO2 did not respond to the increase in delivered O2. In the latter condition, some form of PEEP would be necessary to improve gas exchange by improving functional residual capacity. Patient B has hypercapnic respiratory failure (ventilatory failure) with hypoxemia, but with P(A − a)O2 of 7 mm Hg, which is within the normal range. A pure ventilatory defect is the cause of hypoxemia, and administration of 100% FiO2

