Replacement of Ascending Aorta and Hemi Arch
Nimesh Desai
Joseph Bavaria
Nonelective Indications
Any new-onset acute dissection, rupture, or intramural hematoma generally warrants immediate surgery. The presence of symptoms of chest pain in patients with ascending aortic aneurysms greater than 5 cm is a sign of impending rupture and should also be managed operatively with expediency. Acute severe congestive heart failure secondary to root dilatation and loss of sinotubular junction definition either from rapid aneurysm expansion or chronic dissection also warrants early operative management, although aggressive diuresis and cardiac optimization for 1 to 2 days prior to surgery will lead to fewer postoperative complications.
Elective Indications
Decisions to intervene are based on maximal aortic diameter and growth rate. For degenerative aneurysms in the absence of connective tissue disorders or other cardiac pathology, elective repair is reasonable at an absolute maximal diameter of 5.5 cm. Growth rate of greater than 1 cm per year is generally accepted as a strong indication to proceed with surgery for degenerative aneurysms regardless of diameter. Normalized aortic dimensions to body size provide a more accurate reflection of the aneurysm dimension for an individual patient.
The aortic ratio is calculated as measured maximal aortic diameter divided by predicted diameter for a given age, body surface area. Using this method, elective replacement is warranted at an aortic ratio of 1.5 in an asymptomatic patient without a connective tissue disorder or other complicating factor.
Connective Tissue Disorders, Bicuspid Aortopathy, and Other Considerations
Patients with Marfan syndrome are at higher risk for rupture and the ascending aorta should be replaced prophylactically at a diameter of 4.5 cm or an aortic ratio of 1.3 to 1.4. Patients with Loeys–Dietz may rupture at even smaller diameters and should be
electively repaired at 4.0 to 4.2 cm. Among patients with bicuspid aortic valves, the 2013 Society of Thoracic Surgeons Clinical Practice guideline suggest 5.0 cm (4.5 cm if there is a family history of aortic dissection). In the setting of connective tissue disorders, bicuspid aortic valve or chronic dissection, a growth rate of greater than 0.5 cm per year should warrant repair. Chronic aortic dissections, in which the external aortic wall is supported only by the residual outer third of the medial and adventitial layers, replacement should be performed when aortic diameters reach 4.5 cm or ratio of 1.3 to 1.4 due to the intrinsic weakness of the aortic wall. Pseudoaneurysms, which are frequently from previous aortic suture lines, should be repaired upon diagnosis due to high rupture risk related to their extremely thin walls.
electively repaired at 4.0 to 4.2 cm. Among patients with bicuspid aortic valves, the 2013 Society of Thoracic Surgeons Clinical Practice guideline suggest 5.0 cm (4.5 cm if there is a family history of aortic dissection). In the setting of connective tissue disorders, bicuspid aortic valve or chronic dissection, a growth rate of greater than 0.5 cm per year should warrant repair. Chronic aortic dissections, in which the external aortic wall is supported only by the residual outer third of the medial and adventitial layers, replacement should be performed when aortic diameters reach 4.5 cm or ratio of 1.3 to 1.4 due to the intrinsic weakness of the aortic wall. Pseudoaneurysms, which are frequently from previous aortic suture lines, should be repaired upon diagnosis due to high rupture risk related to their extremely thin walls.
In general, in the setting of other cardiac surgery, ascending aortas with a maximal dimension of 5.0 cm or a ratio of 1.5 should be replaced. For patients with bicuspid valves, the ascending aorta should be replaced at 4.5 cm.
Patients undergoing surgery for ascending aortic aneurysms require a full cardiovascular evaluation prior to surgery. Electrocardiography may show left ventricular hypertrophy or strain or evidence of concomitant coronary artery disease, or previous myocardial injury. Chest x-ray may show enlarged mediastinum with a convex contour of the right superior mediastinum and loss of the retrosternal air space. Echocardiography provides a reliable technique to measure the annular, sinus, sinotubular junction and ascending dimensions as well as grading of aortic stenosis and insufficiency, other valvular lesions, and ventricular function. Contrast-enhanced computed tomography (CT) is the most widely used noninvasive technique for imaging the thoracic aorta. CT scanning provides rapid and precise evaluation of the ascending aorta in regard to size, extent, and location of the disease process. CT scanning detects areas of calcification, and modern scanner accurately identify dissections and mural thrombus. Magnetic resonance imaging (MRI) can provide axial and three-dimensional reconstruction of the ascending aorta with the avoidance of iodinated contrast agents and radiation exposure. Contrast-enhanced MR angiography with gadolinium allows more precise measurements of the aorta and its major branches with images comparable to conventional angiography. Coronary angiography should be performed in all nonemergent patients >40 years of age to evaluate for significant coronary atherosclerosis and lesions with greater than 60% to 70% stenosis should be bypassed. Coronary angiography also helps define the coronary anatomy to identify anomalous or intramural coronary arteries which may complicate root replacement.
Nearly one-third of patients undergoing surgery for thoracic aortic disease have chronic obstructive pulmonary disease. Patients with suspect pulmonary function should have spirometry and room air arterial blood gases. Smoking cessation, antibiotic treatment of chronic bronchitis, and chest physiotherapy may prove beneficial in elective situations. Normal renal function should be ensured with the appropriate blood work, and abnormal results should prompt further investigation. Because unaddressed severe carotid disease is a risk factor for stroke during ascending aortic operations, patients over the age of 65 should have duplex imaging of their carotids. Younger patients with peripheral vascular disease, extensive coronary artery disease, carotid bruits, or history suspicious for cerebral ischemia should be investigated as well. CT or MRI of the thoracic and abdominal aorta is usually indicated.
Anesthetic Considerations
All procedures are performed using central venous access and a pulmonary artery catheter. Location of arterial line for blood pressure monitoring should be discussed with the
anesthesia team preoperatively. If bypass is going to be initiated with the axillary artery, the right radial line will read erroneously high and either the left radial or femoral pressure should be monitored for bypass. Nasopharyngeal and bladder temperature monitors are used. Bilateral near infrared spectroscopy (NIRS) is employed to provide real time estimation of cerebral saturation though out the bypass run. Precipitous drops in cerebral saturations are managed with increasing perfusion pressure and hematocrit to the cerebral circulation. In circulatory arrest cases, EEG monitoring is also employed to ensure EEG silence during interruption of cerebral circulation. Anesthesia management includes fentanyl 25 to 50 μg/kg, midazolam 0.1 to 0.2 mg/kg, isoflurane 0.5% to 1.5%, pancuronium 0.1 to 0.2 mg/kg, and end-tidal concentration in CO2. Aminocaproic acid is dosed initially as an intravenous bolus of 5 g, followed by a maintenance intravenous infusion of 1 g/hr and stopped within 2 hours of patient admission to the intensive care unit. Pharmacologic adjuncts in circulatory arrest cases include 1 g of methylprednisolone, 1 g of magnesium sulfate, 2.5 mg/kg of lidocaine, and 12.5 g of mannitol.
anesthesia team preoperatively. If bypass is going to be initiated with the axillary artery, the right radial line will read erroneously high and either the left radial or femoral pressure should be monitored for bypass. Nasopharyngeal and bladder temperature monitors are used. Bilateral near infrared spectroscopy (NIRS) is employed to provide real time estimation of cerebral saturation though out the bypass run. Precipitous drops in cerebral saturations are managed with increasing perfusion pressure and hematocrit to the cerebral circulation. In circulatory arrest cases, EEG monitoring is also employed to ensure EEG silence during interruption of cerebral circulation. Anesthesia management includes fentanyl 25 to 50 μg/kg, midazolam 0.1 to 0.2 mg/kg, isoflurane 0.5% to 1.5%, pancuronium 0.1 to 0.2 mg/kg, and end-tidal concentration in CO2. Aminocaproic acid is dosed initially as an intravenous bolus of 5 g, followed by a maintenance intravenous infusion of 1 g/hr and stopped within 2 hours of patient admission to the intensive care unit. Pharmacologic adjuncts in circulatory arrest cases include 1 g of methylprednisolone, 1 g of magnesium sulfate, 2.5 mg/kg of lidocaine, and 12.5 g of mannitol.
Circulation Management
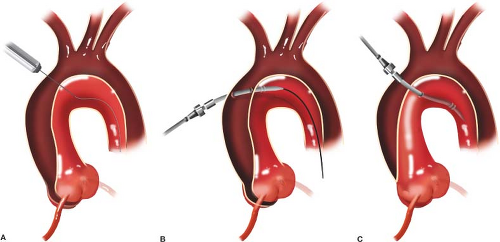
Cannulation strategies vary significantly with individual pathology and the modern cardiovascular surgeon must be proficient in several different techniques. Very large aortas are best cannulated using a Seldinger technique over a wire with TEE guidance. This approach can also safely be applied to acute and chronic type A aortic dissection (Fig. 10.1). In cases where antegrade cerebral perfusion is required, either the ascending aorta may be cannulated directly if using selective direct perfusion cannulas or the right axillary artery may be employed. Right axillary artery cannulation, which has grown in popularity in recent years, should be performed through an 8- or 10-mm Dacron graft anastomosed end to side to the axillary artery as there is risk of dissection from direct cannulation of this friable artery. It is not necessary to distally snare the axillary artery
as “hyperfusion” of the arm is not clinically relevant. The radial/brachial pressure in the right arm does not accurately reflect systemic pressure and should not be relied up except during ACP conditions. In some instances, femoral artery cannulation can be employed, but should be avoided in patients with atheroma in the thoracic aorta by CT scan or TEE.
as “hyperfusion” of the arm is not clinically relevant. The radial/brachial pressure in the right arm does not accurately reflect systemic pressure and should not be relied up except during ACP conditions. In some instances, femoral artery cannulation can be employed, but should be avoided in patients with atheroma in the thoracic aorta by CT scan or TEE.
Deep Hypothermic Circulatory Arrest and Cerebral Protection
Brain and organ protection during periods of circulatory arrest is facilitated by hypothermia. Depending on the complexity of the repair and expected circulatory arrest time, cooling into the moderate range (20° to 28°C) for shorter arrest times <15 minutes and deep hypothermia (14° to 20°C) are used for more complex cases. Cooling is performed maintaining less than a 2° to 3°C gradient between arterial inflow temperature and venous return temperature to ensure even cooling. Nasopharyngeal temperature and bladder temperature which correlate with intracranial temperature and core body temperature, respectively, are also monitored during cooling to guide initiation of arrest.
During rewarming, the bladder, nasopharyngeal, and the systemic perfusion temperatures are monitored. The perfusion is kept at a gradient of not more than 10°C above the nasopharyngeal temperature. This ensures that oxygen demand will not exceed oxygen supply during the interval of cerebral vasoconstriction after DHCA. Avoiding high perfusate temperatures is important and should not exceed 37°C.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree