Repair of Post Infarction Ventricular Septal Defect
David A. Fullerton
Indications
Post infarction ventricular septal defect now complicates less than 1% of acute myocardial infarctions. However, myocardial infarctions complicated by ventricular septal defect tend to be significantly larger than those without, and invariably the infarction involves both the left and right ventricles. Thus, such myocardial infarctions result in significant loss of viable myocardium in addition to the creation of a left-to-right shunt. In the majority of cases, the defect produces significant hemodynamic consequences with heart failure. Without surgical correction, its associated mortality is greater than 90%. Even with surgical correction mortality remains very high, typically between 30% and 50%. But unless a given patient clearly has no chance for meaningful survival, surgical correction is indicated. The procedure should be considered urgent/emergent, as patients will otherwise steadily deteriorate with end-organ dysfunction secondary to cardiogenic shock.
The blood supply of the interventricular septum is derived from the anterior and posterior descending coronary arteries. A post infarction ventricular septal defect arises from acute occlusion of one of these two arteries in the absence of a preformed collateral circulation. Hence, most patients have single-vessel disease, with no prior myocardial infarction or even a history of angina. The majority of cases involve acute occlusion of the left anterior descending coronary artery and result in rupture of the anterior interventricular septum. Posterior defects result from acute occlusion of the posterior descending coronary artery and are associated with a higher mortality.
The diagnosis of post infarction ventricular septal defect is usually made 2 to 5 days following a recent myocardial infarction in a patient with a new pansystolic murmur; it is confirmed with echocardiography. The initial clinical presentation may be deceptive, as the patient may appear to be hemodynamically well compensated. However, the clinical picture will predictably deteriorate as the left-to-right shunt rapidly produces a vicious cycle of pulmonary edema, right ventricular volume overload and failure, hypotension, and inadequate system tissue perfusion. Hence the need for surgical repair.
Contraindications
The procedure may be contraindicated in cases in which there appears to be little chance of meaningful survival of the patient. Such situations will typically be determined by comorbid conditions of the patient or the fact that a given patient is already in extremis. In such situations, the situation may already be futile. A thoughtful, surgical consultation is necessary to make such a determination.
The initial management of the patient with post infarction ventricular septal defect is focused on hemodynamic support. The patient should be admitted to the cardiac or cardiothoracic surgical intensive care unit. To guide therapy, an intra-arterial catheter should be inserted for continuous blood pressure monitoring. A pulmonary arterial catheter should be placed in order that the central venous and pulmonary arterial pressures, cardiac index, and mixed venous oxygen saturation may be continuously monitored. An indwelling urinary catheter should be placed.
Mechanical ventilation may be required if the patient is in respiratory distress secondary to shock or significant pulmonary edema. Intravenous infusion of inotropic and vasoactive agents is typically necessary to combat hypotension. The surgeon must be aware, however, that the etiology of the hypotension is inadequate left ventricular system output secondary to intracardiac shunting. Therefore, administration of vasoconstricting agonists in high dosages in an effort to achieve a higher blood pressure may further compromise poor tissue perfusion. An intra-aortic balloon pump should be placed to lower left ventricular afterload, and thereby decrease the magnitude of left-to-right intracardiac shunting.
In patients in cardiogenic shock, emergency surgical repair is associated with an extremely high operative mortality. Recent experience suggests that outcomes may be improved supporting the patient for a period of time with mechanical circulatory support before surgical repair is undertaken. The preferred form of mechanical assistance is venous–arterial extracorporeal membrane oxygenation (see Chapter 38). This may be quickly established, at the bedside if necessary, by percutaneous femoral artery and femoral vein cannulation. This strategy affords the opportunity to resuscitate end-organ function, particularly renal and pulmonary function, before surgical repair is undertaken.
It may be ideal, but it is not absolutely necessary, to perform preoperative coronary angiography. If coronary angiography is performed, only a small amount of contrast should be used in order to minimize the potential for renal injury. As noted above, either the anterior or posterior descending coronary artery will be acutely occluded and it is not helpful to bypass the infarct-related vessel; the subtended myocardium is already infarcted. Neither short- nor long-term outcomes have consistently been improved with concomitant coronary artery bypass grafting. Nonetheless, some do favor bypassing other significant coronary lesions. This should be done selectively, and only very severe lesions should be bypassed.
Induction of Anesthesia
The induction of general anesthesia must be accomplished with great care. The patient’s blood pressure is dependent upon high sympathetic tone and avid vasoconstriction. Acute loss of such with an aggressive anesthetic induction may result in hemodynamic collapse. Once general anesthesia has been induced, a transesophageal echocardiographic probe should be introduced.
The patient should be positioned supine on the operating table with arms tucked to the side. The patient should be prepped in the standard fashion as for coronary artery bypass surgery.
Cannulation
The procedure is performed through a median sternotomy incision. After the pericardium has been cradled, the patient is systemically heparinized with an activated clotting time of at least 500 seconds. The aorta is cannulated along with standard bicaval venous cannulation. Cardioplegia cannulas are placed in the ascending aorta for administration of antegrade cardioplegia and the coronary sinus for administration of retrograde cardioplegia. Cardiopulmonary bypass is initiated and the left ventricle is then vented though the right superior pulmonary vein. The patient is systemically cooled to a bladder temperature of 28°C. Once the heart fibrillates, the aorta is cross-clamped and cardioplegia is administered in antegrade and then retrograde fashion. The myocardial temperature is monitored in the interventricular septum and maintained below 10°C throughout the period of aortic occlusion. Cardioplegia is administered approximately every 20 minutes.
Technique
Surgical success of the procedure is dependent upon achieving effective closure of the defect. Several techniques have been described, including a direct patch sewn over the defect through a left ventriculotomy or via the right atrium through the tricuspid valve. However, the technique preferred by this author is that of infarct exclusion as described by David. It has been used with the best outcomes and is recommended for the majority of situations. It is described herein for repair of both anterior and posterior defects. If coronary artery bypass grafting is to be performed, the distal saphenous vein graft anastomoses should be performed first. The infarcted myocardium is friable, and great care must be taken to avoid further myocardial damage in the process of exposing the intended targets.
Repair of Anterior Defect
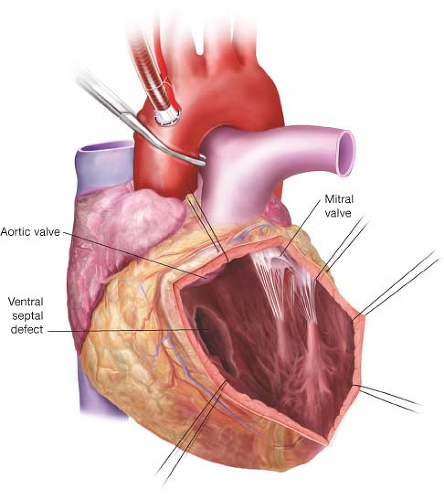
The left ventricle is incised near the left ventricular apex, approximately 2 cm to the patient’s left (leftward) of the left anterior descending coronary artery (Fig. 31.1). The incision is extended through the infarcted anterior wall of the left ventricle toward the base of the heart. Great care is taken to remain approximately 2 cm leftward of the left anterior descending artery. Traction sutures are placed for exposure along the incision to permit visualization of the interventricular septum. This incision provides a view within the left ventricle in which the interventricular septum is to the patient’s right (rightward) and the posteromedial papillary muscle and the mitral valve chordae are displaced leftward.
The infarcted interventricular septum typically has a hemorrhagic appearance and is extremely friable. It is usually very difficult to identify the extent of the ventricular septal defect as the defect is often created in such a way that it courses through the septum in an indirect manner. There may be more than one defect. Attempts to debride the infarcted tissue are discouraged.
The technique of ventricular exclusion offers the advantage that a patch is sewn to viable myocardium, with sutures placed away from the infarcted septum. However, its disadvantage is that the repair is somewhat difficult to visualize in the mind’s eye—it requires the anticipation of a three-dimensional reconstruction of the interventricular septum. A patch of bovine pericardium is placed in such a way as to create a new rightward boundary of the left ventricle. In this way, the patch is used to “exclude” the septum from the left ventricle. Once the repair is completed, the infarcted septum will reside on the right ventricular side of the patch.
 Get Clinical Tree app for offline access 
|


