Renovascular hypertension was first recognized more than 75 years ago by Goldblatt and his colleagues, who produced sustained hypertension in a canine model after gradual reductions in renal artery blood flow using an externally controlled vascular clamp. Subsequent studies have discounted the importance of kidney ischemia per se as the cause of renovascular hypertension, with other hemodynamic signals appearing essential in increasing renin release, the most obvious being a decrease in mean renal artery perfusion pressure and a dampening of arterial flow beyond the renal artery stenosis.
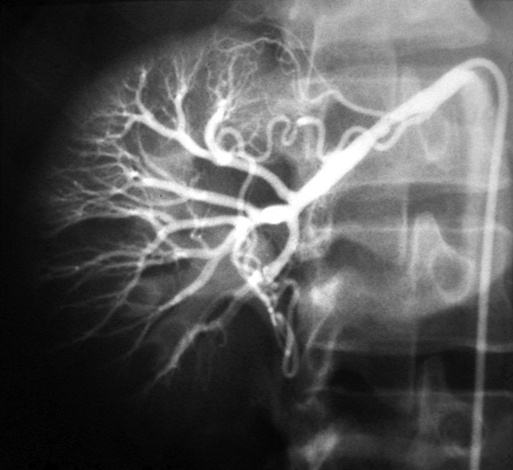
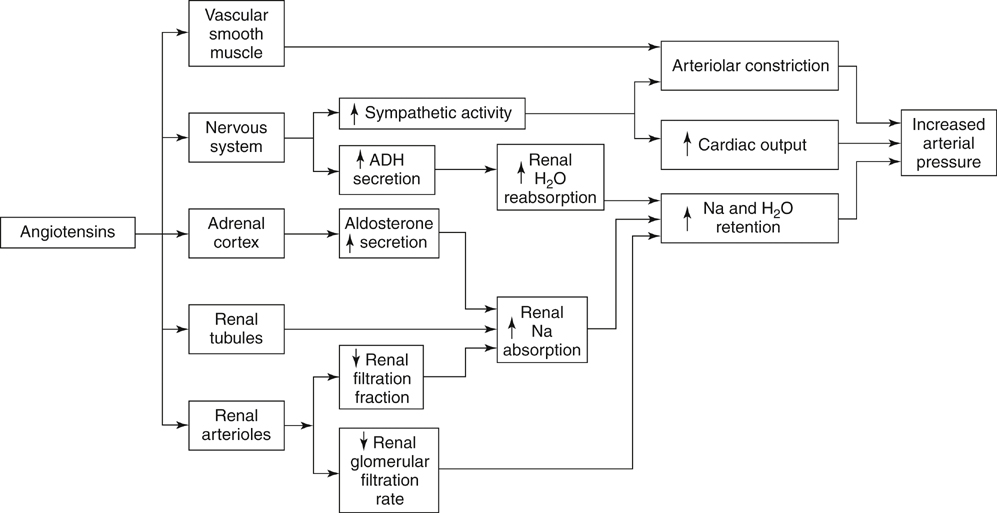
It is generally accepted that a critical renal artery stenosis causes a pressure gradient of 10 to 15 mm Hg, evident by the evolution of collateral vessels circumventing the narrowing (Figure 1). Stenoses of this magnitude are sufficient to cause increased renin release. Renin and its effects on angiotensin and aldosterone are the essential elements causing elevated blood pressures in renovascular hypertension (Figure 2).
Renin is produced in the juxtaglomerular apparatus of the kidney. This anatomic area consists of myoepithelioid cells or granular cells located on the wall of the afferent arterioles; the macula densa, which is composed of specialized tubular epithelial cells in the glomerular hilus at the transition of the loop of Henle to the distal convoluted tubule; and lacis cells located in the region of the efferent glomerular arteriole and the macula densa. Lacis cells are anatomically similar to mesangial cells. The interrelations among the macula densa, glomerular arteriole vasomotion, and renal tubular function are important in understanding renin kinetics.
Renin production and its release from the kidney at the cellular and molecular level is complex. Renal baroreceptors, acting as stretch receptors, affect the release of renin from juxtaglomerular apparatus. Renin release is also affected by changes in pressure at the afferent renal arteriole level, as well as with changes in renal interstitial volume and pressure.
Renin is a proteolytic enzyme that acts on its only known substrate, angiotensinogen. Renin is a single-chain polypeptide with a molecular weight of about 38 kd. It is stored as granules within the juxtaglomerular cells and, in some instances, as granules within the arteriolar wall. Renin has a half-life of 20 to 30 minutes. Extrarenal renin or renin-like enzymes (isorenins) have been identified in the submaxillary salivary gland, uterus, placenta, and brain, but there is no evidence that these substances are functionally important in regulating blood pressure.
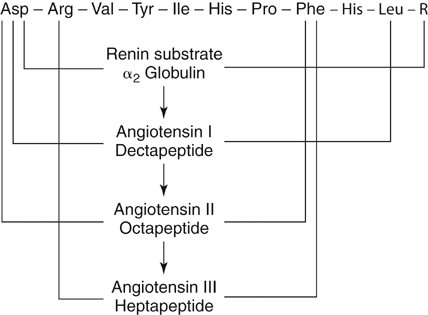
The biochemistry of the renin–angiotensin system is well defined (Figure 3). The primary function of renin appears to be the hydrolysis of angiotensinogen to form angiotensin I. Angiotensinogen, an α2-globulin produced in the liver with a molecular weight of 60 kd, has no vasoactive properties. Expression of the angiotensinogen gene is subject to a variety of physiologic and pathophysiologic stimuli, including steroid hormones, angiotensin II, and sodium loading.
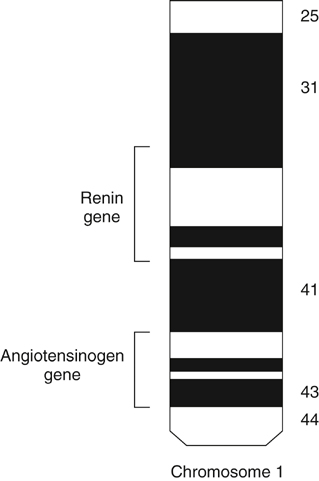
The angiotensinogen and renin genes are located on chromosome 1 (Figure 4). It is of interest that these genes exist in multicellular organisms that have no circulatory system, where they are presumed to have served a very different function than they do in humans. One action that appears to have been preserved during evolution is the proinflammatory property of angiotensin II.
Only gold members can continue reading.
Log In or
Register to continue
Related
![]()



