Chapter 149
Renovascular Disease
Acute Ischemia
Matthew A. Corriere
Based on a chapter in the seventh edition by J. Hajo van Bockel and Jaap F. Hamming
Acute renovascular ischemia is defined as sudden interruption of arterial and/or venous renal blood flow, and may involve either partial or complete ischemia of one or both kidneys. Because the renal arteries are considered “end arteries,” acute ischemia can rapidly progress to irretrievable loss of renal function if it goes unrecognized or untreated. Appropriate clinical management of acute renal ischemia is therefore time sensitive, and assessment of the duration and degree of ischemia is important for decision making related to both medical and procedural intervention. In acute renal ischemia, salvage of a functioning kidney requires a high index of suspicion, rapid diagnosis, and immediate definitive management.
Causes of acute renal ischemia include renal artery thrombosis, embolism, trauma, aortic or renal artery dissection, iatrogenic injury, and renal vein thrombosis (Box 149-1). Evidence supporting specific management strategies for acute renal ischemia is limited and primarily comes from case reports and small cohort studies, perhaps due to the relative rarity of this diagnosis and the urgent nature of clinical planning and management.
Main renal artery occlusion occurring during endovascular aortic aneurysm repair is discussed in Chapter 132. Similarly, other iatrogenic injuries of the renal artery, including complications that occur during angioplasty and stenting, are specifically covered in Chapter 146. Acute renal ischemia associated with aortic dissection is covered in Chapter 138 and detailed discussions of open surgical revascularization are found in Chapter 145. Further discussion on management of renal artery trauma, including penetrating injury, can be found in Chapter 158.
Pathophysiology
Renal ischemic injury is characterized by glomerular collapse and tubular necrosis, resulting in reduced glomerular filtration and loss of tubular function.1 In acute ischemic renal failure, endothelial dysfunction is further induced by reperfusion, which results in excess secretion of cytokines and expression of adhesion molecules that promote leukocyte influx. Reperfusion injury also leads to excessive production of nitric oxide and reactive oxygen species, which leads to further tissue damage and impaired autoregulation of renal blood flow.2
The baseline kidney function and duration of acute warm renal ischemia affect the potential for recovery of renal function and are therefore important considerations when revascularization is being considered.
Baseline Kidney Function
Age and diabetes have been identified as factors associated with increased susceptibility to injury from acute renal ischemia in experimental models.3,4
Duration of Ischemia
In a normal kidney, 1 hour of warm renal ischemia is associated with loss of 70% to 80% of renal function, but complete recovery can occur within weeks; increasing the duration of ischemia to 2 hours diminishes long-term recovery to 30% to 50% of baseline.5 In vivo models of acute, total renal artery occlusion have demonstrated irreversible ischemia after 3 to 4 hours, but this varies substantially by species, with much shorter periods of warm ischemia resulting in irreversible damage in smaller species, such as rats, in which 60 to 90 minutes results in irreversible injury, whereas no recovery has been observed after 120 minutes of warm ischemia in monkeys.6,7 Prolonged ischemia time (>45 minutes) has been identified as a predictor of poor early graft function after living donor kidney transplantation,8 and more than 90 minutes of ischemia has been suggested as a cut-point for recovery of renal function in acute ischemia.9 Technically successful revascularization performed after longer durations of acute ischemia therefore may not be followed by functional recovery, especially when overt renal failure or anuria is already present.9,10
Gradual versus Acute Renal Occlusion
In contrast to acute renal artery occlusion, gradual main renal artery occlusion (e.g., main renal artery stenosis that progresses in severity over months or years) may result in development of collateral circulation that can decrease the ischemic insult. Renal artery collaterals originate from the inferior adrenal, gonadal, ureteral, internal iliac, lumbar, intercostal, and capsular arteries (Fig. 149-1).11–14 Less commonly, collaterals may also originate from the inferior mesenteric artery.15 Prerenal communication may exist at the hilum, whereas intrarenal communication occurs through capsular perforating arterioles. As much as 80% of renal arterial collateral communication may be independent of the main renal artery,7 potentially allowing maintained kidney viability in the setting of main renal artery occlusion (particularly in acute thrombosis of a chronic critical stenosis).13,16
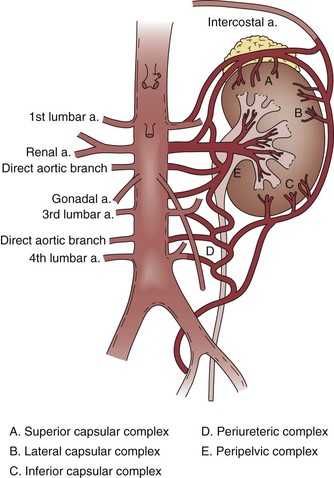
Figure 149-1 Collateral circulation of the kidney. (From Flye MW, et al: Successful surgical treatment of anuria caused by renal artery occlusion. Ann Surg 195:346-353, 1982.)
In a canine model of slow renal artery occlusion that occurred over 7 weeks, collateral circulation developed that was sufficient to maintain both renal viability and life.17 Glomerular collapse and tubular necrosis occur following acute reduction of renal perfusion pressure; in gradual reduction of renal perfusion, however, maintained tubular function (reflected by low fractional excretion of sodium and normal N-acetyl-glucosaminidase excretion) has been observed despite reductions in both the glomerular filtration rate and filtration fraction.1 Reports of functional recovery following periods of main renal artery occlusion exceeding 24 hours underscore the importance of collateral perfusion and demonstrate that potential for kidney salvage is not a function of warm ischemia time alone.18–26 Main renal artery occlusion accompanied by good flow in the renal vein has been suggested as an indicator of collateral arterial flow on duplex ultrasound.26
Renal Vein Thrombosis
Renal vein thrombosis, which may occur primarily or in association with thrombosis of the inferior vena cava, can also produce acute renal ischemia. Renal vein thrombosis may be unilateral or bilateral; as with acute arterial ischemia, the degree of functional impairment and severity of clinical presentation are related to the acuity of venous occlusion and presence or absence of collateral flow.27 When renal vein thrombosis occurs rapidly in the absence of developed collaterals, congestion and edema of the affected kidney can produce pain due to capsular distention and hemorrhagic infarction.28,29 Experimental models have demonstrated that renal injury and blood flow reduction are both more severe with acute venous versus arterial occlusion.30
Clinical Presentation
Symptoms and signs of acute renal ischemia include abdominal or back pain, dyspnea, nausea, vomiting, hematuria, anuria, and acute hypertension.19,21,24,25,31–34 Because many of these symptoms are relatively nonspecific, the differential diagnosis for acute renal ischemia is broad and includes a variety of renal and nonrenal pathologies.
Renal colic with nephrolithiasis is a common initial presumptive diagnosis due to the similarity of presenting symptoms, leading several authors to conclude that an unenhanced computed tomography (CT) scan performed for suspected renal colic should be followed by a contrast-infused study to rule out acute ischemia if no calculi are identified.35–38
Other diagnoses with symptoms similar to renal ischemia include pyelonephritis, renal carcinoma, mesenteric ischemia, cholecystitis or biliary colic, gastritis, splenic infarction, myocardial infarction, and pulmonary embolism.19,25,32,35,39
In a series of patients with embolic renal artery occlusion secondary to atrial fibrillation described by Hazanov et al,35 the majority of patients presented more than 24 hours following onset of symptoms. Huang et al31 reported a median time of 31 hours between symptom onset and presentation to the emergency department for acute renal infarction.
Renal vein occlusion may also present with unilateral renal congestion and tenderness, although the enlarged kidney is rarely palpable in adult patients.28 In a retrospective cohort study of neonates with renal vein thrombosis, the “classic triad” of flank mass, gross hematuria, and thrombocytopenia was only present in 13% of patients.40
Diagnostic Evaluation
Laboratory Tests
Because no single laboratory test is diagnostic for acute renal ischemia, tests must be selected and interpreted in combination with the clinical presentation and physical examination findings.
Laboratory findings associated with acute renal ischemia include leukocytosis, elevated lactate dehydrogenase (LDH), microscopic or gross hematuria, proteinuria, elevated D-dimer, and eosinophilia.* Eosinophilia may be indicative of atheroembolism.42,43 In a series of 20 patients with acute renal ischemia, 95% of patients had an elevated serum LDH, whereas 80% had the triad of flank and/or abdominal pain or tenderness, elevated serum LDH, and proteinuria.31 Only half of patients presenting with acute renal ischemia have elevated serum creatinine36; therefore, a normal serum creatinine does not exclude this diagnosis. In patients with a functional contralateral kidney, asymptomatic unilateral renal ischemia may be identified as an incidental imaging finding, the acuity of which cannot be determined.
Imaging Tests
Imaging plays a critical role in the diagnosis of acute renal ischemia due to the frequently nonspecific nature of the presentation, physical examination, and laboratory findings. The ideal diagnostic imaging test for renal ischemia would be rapid, widely available, noninvasive, inexpensive, and without need for either radiation exposure or contrast administration. Although no imaging technique has all of these characteristics, consideration of these attributes is helpful when choosing the most appropriate imaging test for a given situation.
Computed Tomography
Contrast-enhanced CT is a widely available imaging technique that is rapid and useful for ruling out other soft tissue problems when acute renal ischemia is being considered, and is also useful for evaluating other injuries in the setting of suspected trauma. Although CT results in radiation exposure and requires contrast administration for definitive diagnosis, it is more sensitive than ultrasound and less time consuming than magnetic resonance imaging (MRI), with a reported sensitivity of 80%.35 CT imaging also provides information on aortoiliac anatomy and/or disease that may be relevant when selecting an approach to revascularization. Contrast CT has also been suggested as the diagnostic imaging choice for renal vein thrombosis, and has a sensitivity and specificity that both approach 100%.29 Features of a renal infarct on CT imaging include areas of hypoattenuation with an associated mass effect (Fig. 149-2) or a “cortical rim sign,” which is described as a rim of functioning nephrons supplied by capsular collaterals surrounding an otherwise nonfunctioning kidney.35,36,44,45
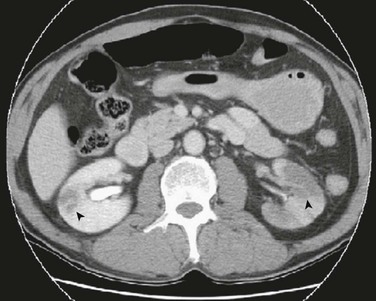
Figure 149-2 CTA demonstrating bilateral renal infarcts. Patient had sudden onset bilateral flank pain with a history of atrial fibrillation. Bilateral wedge-shaped, hypodense lesions (arrowheads) are consistent with embolic renal infarcts. (From Huang C-C, et al: ED presentations of acute renal infarction. Am J Emerg Med 25:164-169, 2007.)
Magnetic Resonance
Renal magnetic resonance angiography (MRA) is capable of anatomic evaluation of renal artery disease with the additional capability of physiologic assessment through flow-dependent imaging.46 Unenhanced MRA is capable of characterizing renal artery disease with reliability similar to CTA, with distinct advantages for evaluating renal segmental arteries and parenchymal disease,47 making it a potentially valuable technique when contrast imaging is contraindicated due to renal dysfunction. Although these advantages have resulted in increasing utilization of MRA for evaluation of chronic renal artery occlusive disease, MRA has not been utilized extensively as a first-line imaging modality for acute renal ischemia. This may reflect practical limitations of MR when rapid imaging assessment is required because MRI is more time consuming and may require extensive postprocessing before interpretation. Compared with CT and/or ultrasound, rapid access to MRI is often relatively limited within emergency department and hospital environments where acute renal ischemia is commonly evaluated.
Ultrasound
By comparison, ultrasound requires neither contrast nor radiation exposure, but it is less sensitive than CT for renal ischemia. Renal infarcts typically appear as wedge-shaped, hypoechoic lesions with absent blood flow on duplex ultrasound (Fig. 149-3). Ultrasound imaging is also technician-dependent and may be challenging in patients with obesity or excessive bowel gas. In a series of 44 patients with renal embolism, only 3 of 27 (11.1%) ultrasound studies were positive.35 In a retrospective review of 10 patients with renal artery thrombosis or embolism, Cai et al48 concluded that color Doppler ultrasonography was useful for detection of large infarcts caused by main renal artery occlusion, but other imaging modalities were needed to confirm smaller infarcts. They also reported decreased renal artery peak systolic velocity as a finding that could be associated with intrarenal embolism. In additional to renal vein thrombus, other ultrasound findings indicative of ischemia due to venous thrombosis may include unilateral kidney edema or congestion.
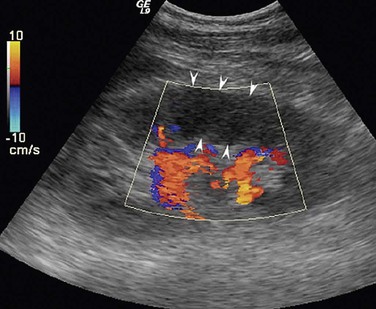
Figure 149-3 Renal infarction demonstrated by duplex ultrasound. Renal infarction caused by arterial embolism appears as a wedge-shaped hypoechoic area with absence of blood flow signal (arrows). (From Cai S, et al: Evaluation of acute renal artery thrombosis or embolism with color Doppler sonography. Clin Imag 32:367-371, 2008.)
Nuclear Renal Scan
Nuclear renal isotope scanning has been suggested as the most sensitive imaging technique for diagnosis of acute renal ischemia, but this modality is time consuming and less widely available. Reported sensitivity of nuclear imaging for acute renal ischemia is 97%,35 and infarcts are characterized by marked reduction of renal blood flow with preserved kidney size.49
Angiography
Although invasive angiography potentially permits endovascular treatment of renal ischemia during the same procedure, use of this method for diagnostic purposes should be limited to situations where other imaging studies are equivocal or the index of suspicion is especially high (i.e., suspected iatrogenic injury).
Renal Artery Embolism
Epidemiology
Risk factors for renal artery embolism include atrial fibrillation, ischemic heart disease, cardiomyopathy, previous thromboembolism, mitral or aortic valve disease, cardiac tumor, atherosclerotic aortic plaque, and paradoxical embolism in the setting of a patent foramen ovale (Fig. 149-4). Renal artery embolism is uncommon, with an incidence among hospitalized patients of 0.007% (or 6.1 per million per year).50
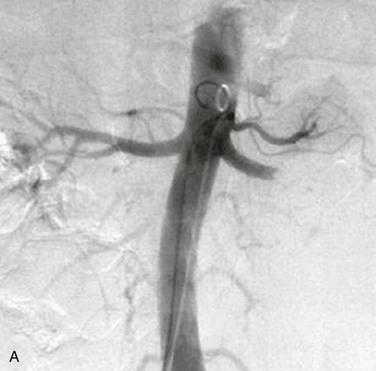
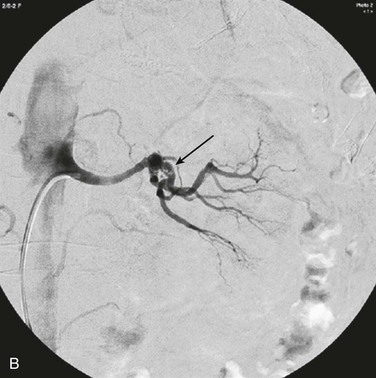
Figure 149-4 Renal artery embolism. A, Contrast angiogram demonstrating left main renal artery occlusion resulting from embolism in a patient with atrial fibrillation. B, Contrast angiogram demonstrating branch renal artery thrombosis resulting from cardiac tumor embolus. (A, From Syed MI, et al: Acute renal artery thrombosis treated with t-PA power-pulse spray rheolytic thrombectomy. Cardiovasc Revasc Med 11:264 e1-7, 2010. B, From Boggetto-Graham L, et al: An uncommon cause of renovascular hypertension. Can J Cardiol 28:397 e1-3, 2012.)
Branches are more commonly occluded by emboli than the main renal artery, making it challenging to distinguish between embolism and thrombosis when branch occlusion with a partial infarct is identified, unless a proximal source can be identified.
Cholesterol crystal embolization (CCE) is a distinct form characterized by multiple, small renal emboli comprised of cholesterol debris. Chronic CCE has an incidence of 6.2 per million people per year; risk factors include male gender, hypertension, and atherosclerosis.51 CCE may also occur as a result of arterial catheterization and/or procedural intervention, especially when multiple instrumentations occur in a diseased suprarenal aorta. CCE is associated with a 30% incidence of progression to dialysis within 2 years and increased mortality.52
Treatment
Heparin anticoagulation should be initiated immediately in patients with embolic renal artery occlusion, once this diagnosis is established or strongly suspected, to prevent local propagation of renal artery thrombus and additional embolic events to other anatomic locations.
Besides anticoagulation, patients with renal embolism also require a thorough evaluation for the source of embolism to determine whether additional treatment (such as repair of proximal aneurysmal disease, thrombectomy, patent foramen ovale repair, or heart valve replacement) is warranted for definitive management and reduction of long-term risk of repeat embolism. More than 20% of patients with renal artery embolism have a history of previous thromboembolism.35,50,53
Although successful management with anticoagulation alone has been described,11,35 revascularization may be considered in patients with acute ischemia who have potentially salvageable renal function, especially in those with bilateral embolism. Conversely, anticoagulation can be used for definitive management in patients with unilateral embolism and a limited potential for renal salvage, (e.g., patients with preexisting medical renal dysfunction). In the report by Hazanov et al,35 more than 80% of patients with renal embolism were managed using anticoagulation without procedural intervention. One-month mortality was 11% in this series; 61% of patients had normal renal function at long-term follow-up, and only 8% required dialysis.
Endovascular Treatment
Catheter-directed thrombolysis is a valuable treatment strategy in acute renal ischemia resulting from arterial embolism, where the occlusion is typically located at the renal artery bifurcation or distal segmental branches (Fig. 149-5).10,32,54,55 Rheolytic thrombectomy can be used in combination with thrombolysis and may permit definitive intraprocedural management without need for placement of a catheter for extended thrombolytic infusion.23,33 Aspiration thrombectomy, angioplasty, and stenting may also be utilized as adjuncts to thrombolysis in the setting of embolic renal artery occlusion.25 In a series of 14 patients with acute embolic renal artery occlusion, Blum et al9 did not observe recovery of renal function in any patients with complete main renal artery occlusion, whereas partial functional recovery was observed in patients with either segmental branch or incomplete main renal artery occlusion.
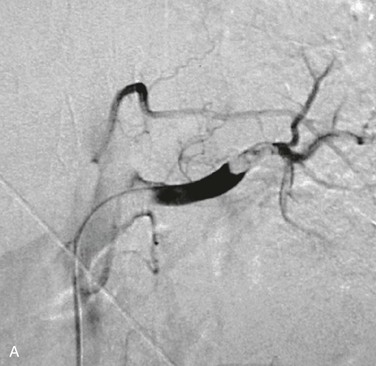
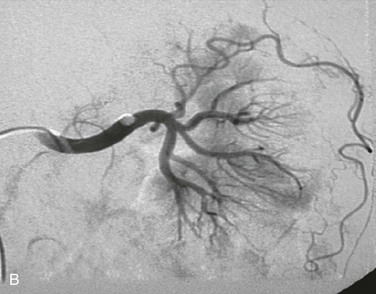
Figure 149-5 Management of renal artery embolism with catheter-directed thrombolysis. A, Distal left renal artery embolus demonstrated by angiography. B, Patent left renal artery demonstrated following catheter-directed thrombolysis. (From Robinson S, et al: Acute renal artery embolism: a case report and brief literature review. Ann Vasc Surg 22:145-147, 2008.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


