Chapter 146
Renovascular Disease
Endovascular Treatment
Matthew S. Edwards, Christopher J. Cooper
Based on a chapter in the seventh edition by Matthew A. Corriere and Matthew S. Edwards
The treatment of renovascular disease is a controversial topic. No high-quality clinical trial or level I evidence exists to support the efficacy of renal revascularization therapy in the treatment of renovascular hypertension or ischemic nephropathy. Nonetheless, renal revascularization remains a commonly applied therapy with endovascular techniques, principally renal artery percutaneous transluminal angioplasty with endoluminal stent placement (RA-PTAS), representing the most common modality of revascularization by a large margin.1–4 Endovascular techniques offer the benefits of decreased morbidity, mortality, patient recovery time, and hospital resource utilization compared with conventional open surgical revascularization.5–10 This chapter provides an overview of the technical aspects involved in the performance of endovascular renal revascularization as well as current data concerning the technical results, clinical outcomes, and associated complications.
Indications and Contraindications
Indications
The indications for renal artery revascularization are controversial in light of the absence of level I data demonstrating the efficacy of such therapy in improving adverse event–free survival among patients with renovascular disease. As described in Chapter 144, two recent clinical trials conducted in Europe, the ASTRAL (Angioplasty and Stenting for Renal Artery Lesions) trial11 and the STAR (stent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by atherosclerotic ostial stenosis of the renal artery) trial,12 and the recently reported CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) trial12a demonstrated no evidence to support the widespread application of renal stents to patients with renovascular hypertension or ischemic nephropathy. Both of the European trials have been criticized for methodologic shortcomings that limit the generalizability of their results and conclusions. Those limitations aside, the data presented in ASTRAL (and to a lesser extent in STAR) strongly suggest that the application of renal revascularization therapy to patients without strong clinical indications (i.e., prophylactic or “drive-by” revascularization) lacks demonstrable benefit for the patient. The results from the CORAL trial, which was more scientifically rigorous and robust, also demonstrated no clinical benefit for the widespread application to patients with renal artery stenosis and significant hypertension with or without associated renal dysfunction.
The initial results from the CORAL trial, also demonstrated no benefit from RA-PTAS in patients with significant hypertension with or without associated renal dysfunction. Although no interactions were observed for bilateral renal stenosis, degree of stenosis, or other clinical factors in terms of clinical benefit; additional analyses are planned for the CORAL data that may be of assistance in identifying patient subgroups more likely to benefit from RA-PTAS. Until those analyses are complete, guidance can be provided by existing consensus documents for severely affected patients or patients not representative of the CORAL study population. In general, those consensus reports state that decisions to perform renal revascularization should be based on the presence of hemodynamically significant renal artery stenosis in the setting of severe, difficult to control hypertension with or without associated renal dysfunction or in the setting of clinical congestive heart failure.14
Hypertensive indications and manifestations of renovascular disease include a spectrum of clinical presentations, such as chronic, severe hypertension that is refractory to pharmacologic management; renovascular hypertension associated with cardiovascular disturbance (especially in the setting of clinical congestive heart failure); and more acute hypertensive emergencies, which refer to acute blood pressure elevations associated with target organ damage, including flash pulmonary edema, hypertensive encephalopathy, myocardial infarction, and acute renal failure. Given the recent findings of ASTRAL and CORAL,11 which demonstrated no reduction in adverse cardiovascular or renal events despite significant blood pressure decreases after RA-PTAS, as well as other large case series demonstrating a lack of survival benefit in association with blood pressure improvement alone,5,7,8,15,16 many authorities on renovascular disease are advocating the application of renal revascularization to cases of truly refractory hypertension and those cases of hypertension complicated by defined end-organ effects.17,18
Renal dysfunction in patients with hemodynamically significant atherosclerotic renovascular disease is commonly referred to as ischemic nephropathy,3,19 although definitive establishment of a causal relationship between anatomic renovascular disease and renal dysfunction can be challenging in an individual patient despite extensive diagnostic testing. The diagnostic evaluation and pathophysiology of atherosclerotic renovascular disease are discussed in detail in Chapter 144. Conditions that would support a causative relationship between anatomically defined renal artery stenosis and disease manifestations are primarily based on reported predictors of response to intervention and include the precipitous decline in renal function during a short time, the severity of hypertension, and the presence of anatomic disease affecting perfusion to the entirety of the functioning renal mass (i.e., bilateral renovascular disease or renovascular disease affecting a solitary kidney).7,20–24 In truth, though, a causative relationship can be proved only by a physiologic response after revascularization. Available data from numerous authors suggest that documented improvement in renal function after renal revascularization is the most important predictor of improvements in adverse event–free survival, suggesting that renovascular disease associated with renal dysfunction may represent the most appropriate contemporary indication for RA-PTAS.5,7,25–27
Contraindications
Contraindications to the treatment of renovascular disease by endovascular means can be broadly classified into anatomic reasons, cases in which open revascularization is optimal, and prophylactic treatment. Anatomic contraindications involve situations in which the disease exists in renal arteries not easily treated with currently available endovascular devices or, more commonly, those arteries unable to be treated with any reasonable expectation of durable result. Renovascular disease extending into the terminal portion of a main renal artery and that involving a very short main renal artery represent two instances in which treatment with currently available technology may be a problem (Fig. 146-1). Renovascular disease existing in the branch arteries beyond the main bifurcation, lesions in multiple small renal arteries (Fig. 146-2), and lesions in children (most commonly hypoplastic lesions) are poor candidates for endovascular treatment because of durability concerns. When encountered, these situations are likely to be best treated with open surgical revascularization unless the patient represents a prohibitive surgical risk. Surgical revascularization is also preferred in patients with indications for renal revascularization who require open aortic surgery.
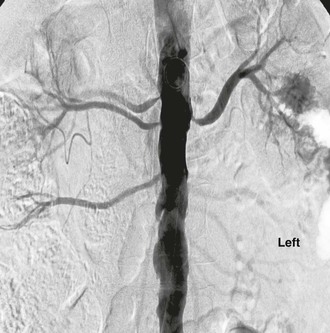
Figure 146-2 Abdominal aortogram demonstrates hemodynamically significant stenosis involving multiple renal arteries.
With regard to prophylactic revascularization, the high rate of technical success and low incidence of periprocedural complications associated with endovascular renal artery revascularization have led to increasingly aggressive application of this technique with rapid growth in utilization in recent years. There is currently no evidence supporting prophylactic renal revascularization (i.e., endovascular treatment of renal artery stenosis in patients with normal renal function and hypertension that is well controlled with conventional medical therapy). Furthermore, the results of ASTRAL,11 the conduct of which approximated prophylactic treatment in a large percentage of its participants, confirmed the lack of efficacy for such an approach in terms of blood pressure control, renal function protection, reduction of cardiac events, or improved survival.
The natural history of atherosclerotic renal artery stenosis is associated with anatomic progression in only 10% to 30% of patients and progression to occlusion in 0% to 7%.28–32 When anatomic disease progression does occur, it is not consistently associated with increases in blood pressure or serum creatinine concentration.30 Considering the benign clinical course of asymptomatic renal artery stenosis in the majority of patients together with the risk of procedure-associated deterioration in renal function with intervention (discussed further later) and the recent results from the CORAL trial, we support a nonoperative, noninterventional management strategy for asymptomatic patients with anatomic renovascular disease and normal renal function and normal or easily controlled blood pressure.
Results
Definitions of Success
Criteria for procedural technical success include reduction in stenosis as assessed by completion angiography and, when endoluminal stenting has been performed, complete coverage of the stenotic lesion by the deployed stent. Post-intervention residual stenosis of less than 30% has been suggested as a threshold for technical success versus failure.33 When contrast angiography demonstrates anatomically successful treatment of the stenotic lesion, these findings should be further confirmed with transduced pressure measurement immediately proximal and distal to the treated lesion. We consider a persistent systolic pressure gradient of 10 mm Hg or more to be suggestive of inadequate treatment and will repeat angioplasty in this setting in the absence of a significant residual stenosis on the completion angiogram.
Whereas technical success implies delivery of the intended treatment with satisfactory anatomic and hemodynamic results, determination of whether patient benefit has been derived requires consideration of the indications for intervention and the clinical responses observed. Approaches to the assessment of hypertension response have evaluated systolic, diastolic, and mean blood pressure and a number of antihypertensive medications both as continuous outcome measures and in a categorical fashion using a combination of blood pressure and medication criteria.33 The categorical definitions generally describe hypertension responses as cured, improved, or failed. Serum creatinine, cystatin C, estimated glomerular filtration rate (eGFR), and renal length and volume all represent continuous outcome measures indicative of renal function response to intervention. Categorization of renal response based on these measures has generally been determined as improved, unchanged, or worsened on the basis of post-intervention change within a predefined range. We have generally used an increase or decrease of 20% or more in eGFR to define improvement or worsening in renal function, respectively, with all other patients categorized as unchanged. Alternative analytic approaches have evaluated the impact of renal revascularization on the rate of decline in renal function, defining worsened or unchanged rates of eGFR decline as failure and attributing procedure-related benefit to patients experiencing improvement, stabilization, or slowed rate of decline in eGFR.
Unfortunately, none of the previously discussed parameters assesses the impact of intervention on patient morbidity or survival. Survival free from dialysis or cardiovascular morbidity represents an outcome that provides the most direct means of assessing the true benefit of intervention and likely represents the outcome of greatest importance in measuring the true value of renal revascularization to the patient.
Early Outcomes
Table 146-1 summarizes outcomes associated with primary RA-PTAS from select contemporary case series of adult patients.* Technical success rates ranged from 88% to 100% and exceeded 95% in the majority of reports. Periprocedural 30-day mortality was 0% to 5% in most recent series (Table 146-1). Procedure-related complications associated with RA-PTAS occurred in 0% to 43% of patients and were most commonly related to arterial access, contrast-induced nephropathy, or atheroembolization. In general, these early results mirror those of ASTRAL and STAR.11,12
Table 146-1
Outcomes of Primary Renal Artery Percutaneous Transluminal Angioplasty and Stenting
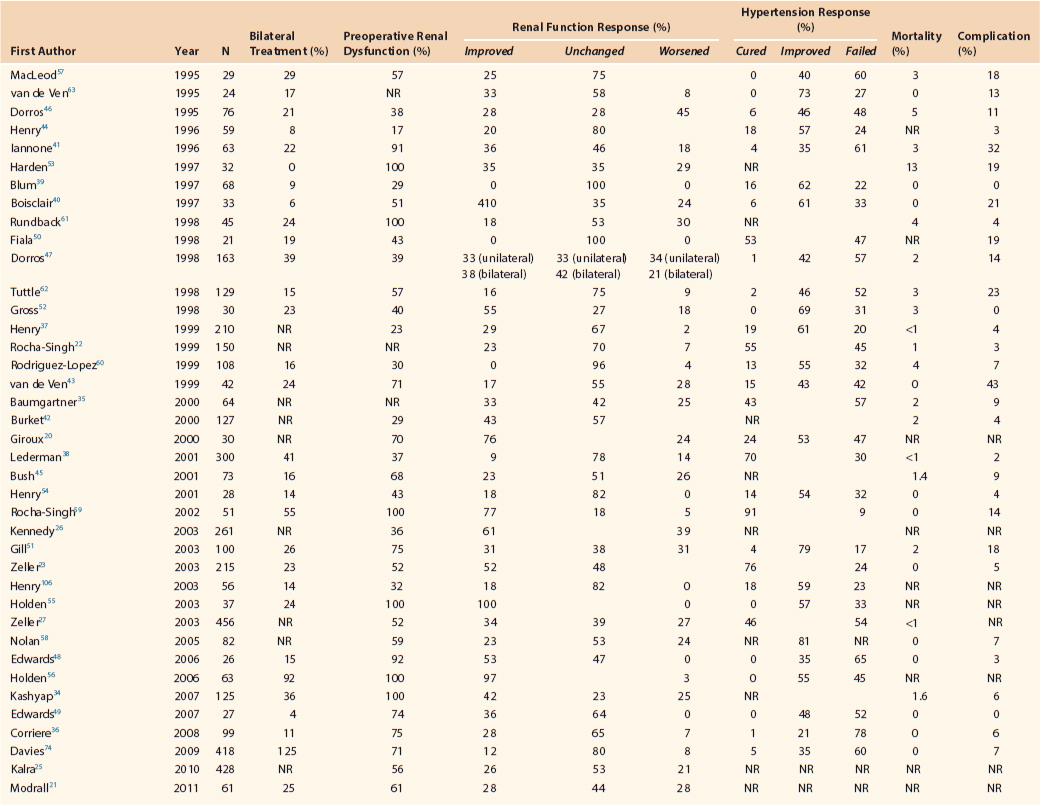
NR, Not reported.
Late Outcomes
Late outcomes associated with endovascular renal artery intervention may be considered in terms of hypertension control, renal function change, anatomic durability, and survival free of dialysis or cardiovascular morbidity. Interpretation of the influence of RA-PTAS on these outcomes is currently challenging, primarily because of limitations of presently available clinical evidence.
Hypertension Response
Long-term hypertension response to RA-PTAS performed for atherosclerotic renovascular disease is most often improvement or failure, whereas cure is uncommon. In the case series data presented in Table 146-1, hypertension improvement was seen in a majority of patients, but failure to improve blood pressure control was also a common outcome. In ASTRAL, no significant improvement in systolic blood pressure was observed when stenting was directly compared with medical therapy. A modest decrease in diastolic blood pressure was observed, and a statistically (although probably not clinically) significant decrease in the number of antihypertensive medications administered was observed.11 In CORAL, a small but significant decrease in systolic blood pressure was observed with no resultant decrease in medications.
Reported predictors of hypertension response have included severity and duration of preoperative hypertension,20,22,23 age of the patient,64 percentage angiographic stenosis,20 bilateral disease,22 female gender,23 and preoperative brain natriuretic peptide level,65 although the brain natriuretic peptide finding is now in question.66 Durable hypertension responses have been reported by several authors,39,46,47,58 but others have observed a loss of initial hypertension response at 6-month post–RA-PTAS follow-up associated with a return to the preoperative number of blood pressure medications.62,67 Given the data from ASTRAL11 and CORAL12a and the lack of association between blood pressure improvements and improved adverse event–free survival reported by multiple authors, there is increasingly limited enthusiasm for RA-PTAS for management of hypertension in the setting of normal renal function. For hypertensive indications, we would recommend limiting intervention for patients with truly uncontrollable and severe hypertension (which is increasingly uncommon given the potency of contemporary antihypertensive medications) and severe hypertension associated with target organ damage.
Renal Function Response
Although several case series have reported improvement as the most frequent categorical renal function response to RA-PTAS,23,52,59 others have observed postoperative deterioration with a frequency equal to or greater than that of improvement,* and renal function was unchanged in the majority of patients after intervention (see Table 146-1). Reported rates of post-intervention improvement versus deterioration in renal function vary widely in these series. The heterogeneous results may be partly attributable to differing patient selection criteria, particularly regarding the percentage of patients with baseline renal dysfunction (because patients with normal baseline renal function have no prospect for improvement through intervention). Weighted averages across series of unprotected RA-PTAS (see Table 146-1) demonstrate renal function improvement in approximately 25% of patients, with a roughly equal proportion experiencing post-intervention decline.
Rather than assessing renal function response as mean or categorical change in outcome measures, several studies have instead analyzed renal responses to RA-PTAS using breakpoint analysis based on the slope of either eGFR or reciprocal of serum creatinine concentration, with categorization of effect as improvement, stabilization, or failure.33 Whereas these studies have demonstrated that RA-PTAS can reduce the rate of renal function decline,34,53,59,67 interpretation of clinical benefit based on breakpoint analysis is controversial. Reciprocal creatinine analysis was employed in ASTRAL,11 and RA-PTAS demonstrated no benefit in slowing the rate of renal function decline relative to the use of best medical management alone. One interesting finding from ASTRAL that deserves to be stressed was the observation of a much lower rate of renal function decline in the medical therapy arm than expected. This most likely represents the effects of ever-improving medical management on the natural history of atherosclerotic renovascular disease.
Case series data do suggest, though, that demonstrated improvement in renal function after renal revascularization does impart benefits for the patient with regard to survival. Hansen et al7 observed improved dialysis-free survival after open surgical revascularization in patients with early postoperative renal function improvement, but no similar survival effect was noted in patients with unchanged renal function. Similar associations with renal function improvement have also been demonstrated after RA-PTAS.25,26,34,68 Although patients with increases in postoperative eGFR (or decline in serum creatinine concentration) appear to experience a survival benefit, clinical benefit resulting from stabilization of renal function or reduction in rate of decline is not clear. We therefore believe that in interpreting either categorized change in eGFR or breakpoint analysis data, patient benefit can be assumed only for patients with improvement, whereas presuming benefit in patients with unchanged or stable renal function may be conceptually flawed. Reported predictors of renal function response to RA-PTAS have included bilateral disease,27 elevated baseline serum creatinine concentration,23,27 higher levels of chronic kidney disease,7,25 rapid preoperative decline in renal function,21,34 impaired left ventricular function,23 absence of metabolic syndrome,69 smaller pre-revascularization renal parenchymal volume,70,71 and improved renal volume after revascularization.71,72
Renal Artery Restenosis after Intervention
Restenosis after RA-PTAS has been defined on the basis of angiography,38,43,62 duplex ultrasound,58 or both.51,60,73 Reported rates of restenosis after RA-PTAS range from 5% to 66% and vary with duration of follow-up and criteria for repeated imaging.† Restenosis is more common in women and in patients exhibiting metabolic syndrome74 and less common in patients treated perioperatively with statins.6 Other factors that may occasionally contribute to restenosis include misplacement of the stent, most frequently inadequate proximal coverage of an ostial lesion, and stent fracture.
Anatomically identified recurrent stenosis must be considered in combination with clinical manifestations in formulating a management strategy. Conversely, acute worsening of hypertension or decline in renal function should prompt suspicion of disease recurrence. Lederman et al38 identified recurrent disease twice as frequently when anatomic imaging was performed in the setting of clinical suspicion as opposed to routine post-intervention screening (42% vs 21%, respectively). The relatively frequent occurrence of recurrent disease should be considered when primary intervention is being contemplated, particularly in asymptomatic patients, and raises additional concerns about the long-term benefits achieved in patients with unchanged renal function after RA-PTAS.
Survival
Long-term survival data for patients undergoing RA-PTAS are presently limited to retrospective studies and limited data from ASTRAL.11 Dorros et al47 observed a 74% 3-year survival rate after RA-PTAS; although renal failure was not a frequent cause of death, they observed decreased survival among patients with both baseline renal dysfunction and bilateral disease. Similar overall survival was reported by Kashyap et al,34 who also observed a 63% rate of dialysis-free survival; lack of post-intervention improvement in renal function was associated with subsequent dialysis in that series. Similar relationships between baseline renal dysfunction, renal function response, and survival have been described by Kennedy et al.26 Survival benefit associated with angiotensin-converting enzyme treatment75 and statin use after RA-PTAS has also been described,26,76 whereas preoperative congestive heart failure, coronary artery disease, and chronic obstructive pulmonary disease are additional factors that adversely affect post-intervention survival.26,38,76
Similar data have come from ASTRAL and STAR.11,12 In ASTRAL,11 5-year survival was 58% for the entire study sample, with no improvement demonstrated for those undergoing RA-PTAS. Improvement in diastolic blood pressure and reduced medication use observed for the stent arm participants were not demonstrated to have any effect on survival. As previously stated, no differences were observed in renal function, rate of renal function decline, or systolic blood pressure. In the results from STAR,12 2-year survival was slightly in excess of 80%, with no differences observed according to post-revascularization blood pressure control or renal function.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree