Renal Artery Stenosis: Surgery
ARSALLA ISLAM and MATTHEW S. EDWARDS
Presentation
A 58-year-old male with a long-standing history of smoking and hypertension is admitted to the hospital with worsening renal function. He is compliant with his four-drug antihypertensive regimen after an episode of flash pulmonary edema that resulted in hospitalization 8 months ago. At that time, his blood pressure was as high as 230/110 mm Hg. His current blood pressure is 150/98 mm Hg. His serum creatinine level is 3.0 mg/dL and was measured at 2.1 mg/dL 6 months ago. His physical examination is unremarkable.
Differential Diagnosis
The differential diagnosis for surgically correctable severe, secondary hypertension includes renovascular hypertension, pheochromocytoma, Conn’s syndrome (aldosterone-secreting adenoma), Cushing’s syndrome, and coarctation of the aorta.
Discussion
Renovascular disease (RVD, renal artery stenosis [RAS] or occlusion) has a prevalence of approximately 7% in older American adults. The prevalence of RVD is increased in certain patient subgroups. RVD should be suspected as a potential contributory factor in patients with hypertension that is severe, refractory to multidrug therapy, or associated with renal dysfunction. Hypertension that presents at the extremes of age should also be suspected of having a renovascular origin.
Potential findings on history and physical and/or laboratory evaluation include an abdominal bruit, retinopathy, unexplained hypokalemia, or a history of congestive heart failure or pulmonary edema. No single feature or test is diagnostic for RVD-associated hypertension. However, in those patients with the above findings, a noninvasive assessment of the renal vasculature is warranted.
Renal duplex ultrasonography has emerged as the initial diagnostic study of choice for RVD. A renal artery peak systolic velocity (RA-PSV) exceeding 2.0 m/s is associated with stenosis greater than 60% on angiography. The ratio of renal artery to aortic peak systolic velocity is also a useful diagnostic parameter, with a ratio greater than 3.5 strongly associated with stenosis of greater than 60%.
Unfortunately, once RVD is identified in combination with severe hypertension or excretory renal insufficiency, discriminating predictors of blood pressure and renal function response to revascularization therapy are lacking. Renal vein renin analysis and measurement of the renal artery resistive index have been used to try and predict the clinical response to revascularization, but the supporting data for these modalities are inconsistent.
Use of a renal vein systemic renin index has been proposed to predict hypertension response. Lateralizing results from selective renal vein renin assays are useful to guide management in selected cases of unilateral renal artery disease, but this test is poorly tolerated by patients, difficult to standardize and reproduce from a practical standpoint, and extremely expensive. Furthermore, renal vein renin analysis is of no utility in cases of bilateral RVD, which accounts for roughly half of all cases. Given these practical limitations, we rarely use renal vein systemic renin index in our practice.
Renal artery resistive index has also been purported as a predictive tool for functional response following renal revascularization. The renal resistive index (RRI) is calculated as 1 − (RA-EDV/RA-PSV). Prior published research by our group and others has demonstrated associations between RRI less than 0.8 and favorable blood pressure and renal function responses. Furthermore, RRI greater than 0.8 has been associated with impaired long-term survival and medical/parenchymal disease of the kidney. Unfortunately, the aforementioned data have not been widely reproducible, and the use of RRI as a predictive tool is not an accepted standard of practice.
Renal Duplex Sonography Results
Renal duplex sonography of the patient’s left renal artery demonstrates RA-PSV of 2.83 m/s proximal, 0.79 m/s mid, and 0.25 m/s in the distal renal artery with a renal-aortic ratio of 4.6. Right renal artery is occluded. Left kidney measures 12.0 cm in length, and the right kidney measures 5.5 cm in length.
Discussion
In this patient, renal duplex sonography was chosen as the initial diagnostic modality. Depending on available expertise, other modalities can be used in the diagnostic evaluation of RVD, such as renal scintigraphy, magnetic resonance angiography, spiral computed tomographic (CT) angiography, and catheter-based digital subtraction angiography. We strongly feel that catheter-based digital subtraction angiography no longer represents a diagnostic modality, but rather represents part of the therapeutic treatment of RVD, except in cases where a definitive diagnosis of RVD is required and not obtainable using the other described methods.
Renal Arteriography
Renal Arteriography Report
Selective renal artery catheterization with arteriography was then performed for this patient using carbon dioxide as the contrast agent. A single angiogram using dilute iso-osmolar contrast was performed once the optimum image orientation was defined using carbon dioxide. The presented angiographic images demonstrate a left RAS of greater than 80% with a very short main renal artery. An occlusion of the right renal artery was demonstrated without evidence of distal reconstitution or any discernible nephrogram. We did not feel that these findings did not represent appropriate anatomy for endovascular therapy, and the procedure was terminated (Fig. 1).
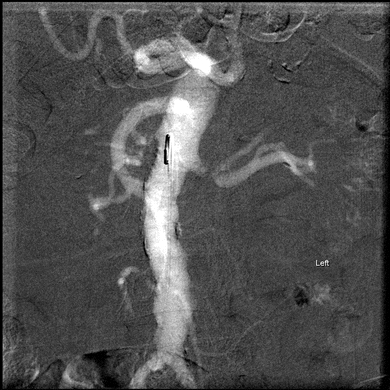
FIGURE 1 Shows carbon dioxide aortogram with nonvisualization of the right renal artery and a left RAS.
Discussion
In patients with hypertension and RVD diagnosed by angiography, the decision to proceed with surgical intervention is based on multiple factors: age, anatomic issues, severity of hypertension, medical comorbidities, and presence of ischemic nephropathy. In certain cases, functional studies may be useful in determining the utility of revascularizing kidneys with questionable function. Our group routinely uses nuclear renography to assess small kidneys for the presence of function prior to revascularization. These nuclear studies can be performed using a variety of isotopes. In general, if a small kidney provides less than 10% of the patient’s renal function, we do not plan to revascularize. In the patient presented, his right kidney did not demonstrate any significant radioisotope uptake or excretion.
Diagnosis and Recommendations
The options for renal revascularization include percutaneous angioplasty or surgical revascularization in patients with RVD, hypertension refractory to medical management, and/or impaired excretory renal function. In this patient, the decision is made to proceed with surgical revascularization of the left kidney as there was no safe way to perform angioplasty and stenting without risking occlusion of a major branch of the renal artery. Given the proximity of the lesion to the main branch point of the renal artery and the extended warm ischemia time that would have been required if a complex anastomosis or multiple anastomoses were required, a decision was made to proceed with renal artery endarterectomy. This was performed using a left subcostal incision with a medial mobilization of the viscera to broadly expose the aorta, left renal artery, and left kidney. The endarterectomy was performed using a longitudinal arteriotomy extending across the aorta onto the larger of the two main branches of the left renal artery. Following bovine pericardial patch angioplasty of the arteriotomy, an intraoperative duplex exam was performed demonstrating a normal low resistance flow pattern with a RA-PSV of 1.4 m/s and no turbulence (Fig. 2).
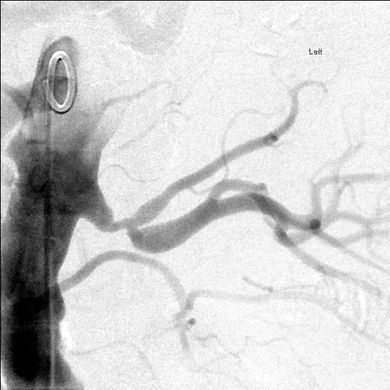
FIGURE 2 Shows stenosis at the branch point of the early bifurcation of the left renal artery.



