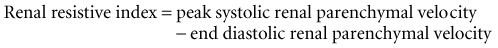41 Renal Artery Stenosis
 Introduction
Introduction
Atherosclerotic renal artery stenosis (ARAS) is associated with chronic renal ischemia as well as increased cardiovascular morbidity and mortality. The primary aim of renal revascularization therapies is to improve blood pressure control, salvage renal function, and reduce cardiovascular risk. However, while technical advances in the endovascular treatment of ARAS have improved dramatically in the past decade, allowing patients with more extensive ARAS to be considered for revascularization, the indications for treating these patients remains controversial and highlights the major divergence in practice between physicians regarding the appropriate treatment for patients with ARAS.1 Recent prospective randomized clinical trials, both small and large, from Europe have failed to demonstrate the clinical benefit of renal revascularization in reducing cardiovascular events and/or renal outcomes compared with medical therapy alone.2–4 Determining the essential role of renal revascularization and appropriate patient selection is now the central issue among physicians, as consensus regarding the general technical aspects of renal stent revascularization has reached a general level of acceptance among interventionalists. Finally, the recent emergence of percutaneous renal sympathetic denervation as a safe and effective technology in the management of drug-resistant hypertensive patients has highlighted the important role of the sympathetic nervous system in hypertension.5 As such, ongoing large clinical trials in the treatment of hypertension and renal insufficiency in these patient populations, with and without ARAS, will ensure that renal interventional therapies for hypertension control will remain an exciting area of clinical research.
 Epidemiology and Natural History
Epidemiology and Natural History
ARAS is the most common secondary cause of hypertension; left untreated, it may progress to renal dysfunction. The prevalence of end-stage renal disease in the United States is 372,407 patients per year, with approximately 100,000 new cases diagnosed each year;6 2.1% of these new cases are thought to be due to ARAS.7 ARAS is reported in 0.5% to 5%8 of all hypertensive patients and 45% of patients with severe or malignant hypertension.9 The prevalence of ARAS increases with age, especially in patients with diabetes, hypertension, coronary artery disease, or aortoiliac occlusive disease.10 A population-based study reported a prevalence of renal artery stenosis (RAS) (>60% stenosis) to be 6.8% in patients above age 65.11 In a Mayo Clinic series, more than 19% of patients with coronary artery disease and hypertension were found to have >50% stenosis of the renal arteries.12 As such, the risk factors for the development of ARAS are similar to those of coronary artery disease. Similarly, stroke and peripheral arterial disease are highly prevalent in patients with end-stage renal disease on hemodialysis. ARAS is progressive in 36% to 71% of patients with this condition, and 39% of patients with >75% ARAS progress to complete occlusion within 3-year follow-up.13 In a prospective study of 84 patients with at least one abnormal renal artery, progression of RAS was reported to occur at a rate of approximately 20% per year.14 Unfortunately, it is difficult to identify the subset of patients who will progress to renal failure. While 90% of renal artery lesions are atherosclerotic, the remaining 10% result from other etiologies. Fibromuscular dysplasia (FMD) is the second most common cause of RAS and results in fibrous thickening of the intima, media, or adventitia of the arterial wall. FMD is more common among women between the ages of 15 and 50 and is recognized by its beaded appearance on angiography.15 Other less common causes include trauma, dissection, external compression with tumor or mass, thromboemboli, renal artery aneurysms, neurofibromatosis, vasculitis, retroperitoneal fibrosi, and radiation-induced stenosis.16
 The Pathophysiology of Renovascular Hypertension and Ischemic Nephropathy
The Pathophysiology of Renovascular Hypertension and Ischemic Nephropathy
The renal vasculature is richly innervated by sympathetic afferent and efferent nerve fibers which controls renovascular resistance, and the resultant increase in renin release.17 Several inputs control efferent renal nerve activity, such as aortic and carotid baroreflexes18 and cardiac stretch receptors.19 Renal nerves often receive greater sympathetic activation than others, especially in the presence of essential hypertension.20 This disproportionate increase in renal sympathetic activity results in increased renovascular resistance and increased plasma renin activity; it also promotes the retention of sodium and water.21 The afferent renal nerves contribute to the pathogenesis of renovascular hypertension by increasing activation of the sympathetic nervous system.17 In unilateral ARAS, decreased blood flow through the affected kidney results in increased production of rennin, which, in turn, cleaves angiotensin to produce angiotensin I, which is converted to angiotensin II. Angiotensin II is a direct vasoconstrictor that stimulates aldosterone secretion and results in sodium reabsorption. The retained salt and water is then excreted by the unaffected kidney, producing a rennin-dependent hypertensive state. In bilateral ARAS or ARAS in a solitary functioning kidney where there is no ability to sense an elevated blood pressure, a pressure natriuresis does not occur, resulting in volume expansion. This, in turn, results in the suppression of renin activity. These patients become highly dependent on angiotensin II for glomerular filtration. Angiotensin II maintains the efferent arteriolar tone of the glomeruli. When angiotensin converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) are administered, the efferent arteriolar tone is no longer maintained and glomerular filtration is decreased, resulting in renal insufficiency. Sodium restriction and diuresis convert bilateral RAS to a renin-mediated form of hypertension.22
 Diagnostic Tests and Imaging
Diagnostic Tests and Imaging
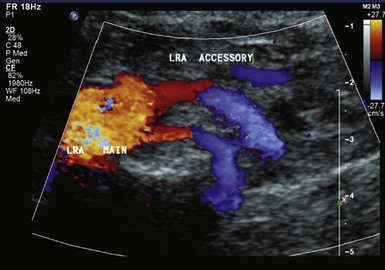
Duplex ultrasound, computed tomography angiography (CTA), and magnetic resonance angiography (MRA) are three contemporary noninvasive imaging modalities used to diagnose RAS. Other tests can be used to assess the potential physiological effect of RAS. Duplex ultrasonography is a useful noninvasive test to screen for RAS, with sensitivity between 75% and 98% and specificity between 87% and 100%.23 Its sensitivity in identifying accessory renal arteries is 67% (Fig. 41-1). Duplex ultrasonography can produce images of the renal arteries, assess blood-flow velocity and pressure waveforms, and measure kidney size without contrast or radiation exposure (Figs. 41-2 and 41-3). Estimation of renal artery percent diameter stenosis is based on the renal artery velocity and renal artery to aortic velocity ratio (Table 41-3), and anti-hypertensive medications do not interfere with duplex imagining. It can also provide information regarding renal parenchymal disease, tumors, and calculi. It is less expensive than CTA or MRA, can be used for renal artery stenting surveillance, and can be easily performed at the patient’s bedside. Unfortunately, early studies of this technology reported a 10 to 20% rate of failure due to operator inexperience, patient obesity, or bowel gas.23 The test may be time consuming when done by inexperienced technologists.
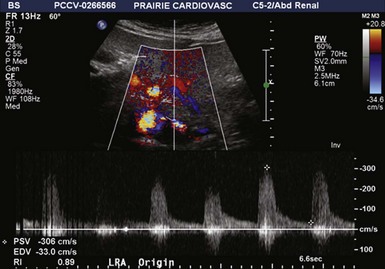
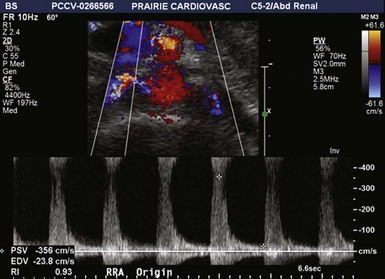
Figure 41-2 Bilateral renal artery stenosis of 60% to 99% on duplex ultrasonography in a 69-year-old woman.
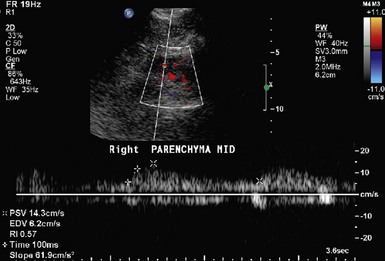
Figure 41-3 Resistive index (RI) and prolonged acceleration time (≥100 msecs, white arrow) in a patient with 60–99% RAS.
The renal resistive index (RI) is a commonly used measure of resistance to arterial flow within the renal vascular bed and is calculated during the duplex ultrasonography (Fig. 41-1).
Peak Systolic Renal Parenchymal Velocity
An elevated RI is considered to be an indicator of nephrosclerosis and intrinsic kidney disease. Radermacher et al. used an RI >0.8 as the sole screening tool to identify a patient subset that may have a less than optimal response of blood pressure control as well as improved renal function with renal artery stent revascularization.24 However, these findings were refuted by other studies suggesting improved renal function and blood pressure control after successful renal artery stenting.25,26 Therefore RI should not be considered the sole parameter in deciding whether a patient will have a clinically favorable response to percutaneous renal revascularization.
Magnetic Resonance Angiography and Computed Tomography Angiography
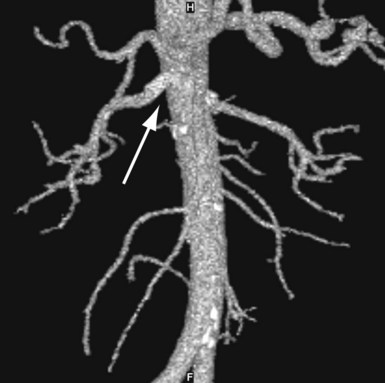
MRA and CTA, both excellent noninvasive techniques to evaluate patients for possible renal pathology, are now widely available. If duplex ultrasonography is nondiagnostic, these modalities can be used to confirm the findings of duplex ultrasound and used with patients whose anatomy is unfavorable for invasive angiography; however, CTA does require the use of iodinated contrast. Both CTA and MRI can provide additional visualization of the aorta, accessory renal arteries, and renal parenchymal anatomy. MRA requires no contrast but cannot be used in patients with ferromagnetic devices or those who are claustrophobic. Stented vessels cannot be adequately evaluated by MRA. Grobner and Marckmann postulated the causative role of gadolinium used in MRA in the development of nephrogenic fibrosing dermopathy.27,28 Since then, the use of MRA has been restricted to patients with serum creatinine <1.5 to 2.0 mg/dL. The sensitivity and specificity of MRA are 62% and 84%, respectively.29 The drawbacks of CTA include large contrast load and nephrotoxicity, along with the radiation exposure. The sensitivity and specificity of CTA are 64% and 92%, respectively.29 CTA can also be used for stent surveillance if duplex ultrasonography is nondiagnostic (Fig. 41-4).
 Functional Assessment of Renal Artery Stenosis
Functional Assessment of Renal Artery Stenosis
Brain naturetic peptide (BNP) is secreted by ventricular myocytes in response to increased myofibril stretch. Its production is also stimulated by angiotensin II, which is elevated in patients with RAS, and by hypertension. BNP, in turn, antagonizes plasma renin activity and promotes diuresis and sodium excretion. Silva et al. studied the role of BNP level in patients with RAS and hypertension.30 In this small study, baseline BNP was elevated in patients with severe ARAS. A significant blood pressure response to renal stenting was seen in patients with elevated baseline BNP. Therefore this test may be useful in identifying patients with renovascular hypertension who would be likely to respond to stent revascularization; however, larger confirmatory studies are required to prove this. Radionuclide imaging has also been historically used to diagnose unilateral RAS. With this technique, a detector gamma camera is used to assess baseline renal flow by injecting a radionuclide tracer (99mTc diethylene triamine pentaacetic acid [DTPA]). Repeat scans are performed after administering the ACEI captopril, which decreases renal function on the ipsilateral side of RAS, while the uptake of the unaffected side is either normal or increased. The reported sensitivity and specificity are 90% and 93%, respectively.31,32 The wide availability of duplex ultrasound, CTA, and MRA has resulted in the near elimination of this test to diagnose RAS. It is occasionally utilized for cortical functional assessment of the ipsilateral and contralateral kidney in the setting of renal atrophy and RAS or for cortical functional assessment of a horseshoe kidney with RAS. Plasma renin activity or plasma renin assay (PRA) has also been used as a screening test for renovascular hypertension. Plasma renin levels have a low specificity in the diagnosis of RAS; however, the concomitant use of an ACEI improves the specificity of PRA levels. PRA is measured at baseline and 1 hour after oral administration of 50 mg of captopril. Unfortunately the sensitivity ranges widely, between 34% and 100%, and the specificity varies from 80% to 90%.33,34 In patients with bilateral or unilateral RAS and a solitary kidney, the sensitivity and specificity is this assay is much lower because the resultant volume overload suppresses PRA levels. Assessment of renal vein renin level is another test for diagnosing renovascular hypertension. This is performed using a catheter to compare the renin levels of both kidneys. Although it may identify blood pressure responders to endovascular intervention or surgery, it has fallen out of favor owing to the invasive nature of the test. Therefore the measurement of plasma renin and renal vein renin activity is seldom needed in contemporary diagnosis and management of RAS because of the availability of noninvasive imaging modalities.
Identification of At-Risk Patients and Indications for Renal Artery Revascularization
The presence of ARAS should be suspected in patients with resistant hypertension and/or progressive renal insufficiency who have coronary artery disease (CAD) and/or peripheral arterial disease (PAD). Specific clinical clues are listed in Table 41-2. The preprocedural identification of the patient with poorly controlled hypertension who is likely to benefit from renal stent revascularization is challenging. The recommendations of the American College of Cardiology/American Heart Association (ACC/AHA) for the management of PAD in peripheral arterial disease, including revascularization in RAS, are as listed in Table 41-1.35 Appropriate indications for renal revascularization include unilateral RAS, bilateral RAS, or RAS involving a solitary functioning kidney in patients in whom blood pressure cannot be adequately controlled with maximal tolerable doses of at least three antihypertensive medications of different classes or if side effects of the antihypertensive medication prohibit sufficient control.36 In patients with unilateral RAS with normal renal function and well-controlled hypertension, revascularization may not be required; instead, close follow-up for potential loss of pharmacological control and accelerating hypertension and/or a potential decline in renal function may suffice. In these instances, a renal duplex ultrasound should be considered. Patients who have undergone successful stent revascularization are often prescribed antiplatelet and statin therapies. The routine use of clopidogrel in combination with aspirin after successful stent revascularizaton has not been adequately evaluated; however, most investigators use this combination in light of other indications (i.e., drug-eluting coronary stent implantation). Statins (HMG-CoA reductase inhibitors) are frequently given to patients with ARAS; these agents may slow the progression of renal atherosclerosis after renal revascularization and in some instances induce plaque regression.37 Cheung et al. found a reduction of ARAS progression from 30% to 6% in patients without and with statins, respectively. They also found 12 of 79 patients exhibited signs of disease regression.37 Therefore statins should be considered in all patients with established dyslipidemia and ARAS.
TABLE 41-1 Duplex Ultrasound Criteria for a Diagnosis of Renal Artery Stenosis
| Indication | Classification of Recommendation | Level of Evidence |
|---|---|---|
| Asymptomatic bilateral or unilateral RAS to a solitary kidney | IIb | C |
| Accelerated hypertension | IIa | B |
| Resistant hypertension | IIa | B |
| Hypertension and unexplained unilateral small kidney | IIa | B |
| Hypertension with intolerance to antihypertensive medications | IIa | B |
| Progressive kidney disease and bilateral RAS or RAS to a solitary kidney | IIa | B |
| Chronic renal insufficiency and unilateral RAS | IIb | C |
| Recurrent, unexplained CHF or sudden unexplained pulmonary edema | I | B |
CHF, congestive heart failure; RAS, renal artery stenosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree