Dissection is a potential complication of endoluminal instrumentation of the renal artery. The risk of dissection increases as the complexity of instrumentation progresses from simple selective renal artery catheterization for diagnostic imaging, to balloon angioplasty, and stent or covered-stent insertion. It is difficult to establish an accurate incidence of this complication because endpoint definitions vary and reporting standards have not been established. Therefore it is likely that many small, limited, clinically silent dissections resulting from endovascular procedures are not reported. Nevertheless, studies from about a decade ago reported rates of dissection less than 1% with selective renal angiography, 2% to 5% with renal artery angioplasty, and 5% to 7% with renal artery stenting.
The risk of endovascular procedure–induced dissection is related to the anatomy of the renal artery (diameter, tortuosity) and the nature of the underlying disease. In comparison to other aortic branches, the renal arteries are smaller in caliber, shorter, more mobile, and more tortuous in both the craniocaudal and anteroposterior planes. Each of these anatomic features increases the risk of dissection during instrumentation. Other anatomic features that influence the risk of dissection include aortic tortuosity (impairs tracking of catheters and devices) and prior pararenal aortic or renal artery surgical procedures (scar tissue fixation prevents the renal arteries from deforming to accommodate the catheters and delivery systems). Dissection is also more likely to occur during instrumentation of arteries with complex atherosclerotic lesions, but it is relatively less likely to occur during instrumentation of arteries involved by fibromuscular dysplasia (FMD). In reviewing 22 reported cases of endovascular procedure–induced renal artery dissections, Smith and coworkers noted associated atherosclerosis in 44% and FMD in only 18%.
Endovascular procedure-related renal artery dissection is usually unilateral because it is confined to the instrumented artery, involves renal artery branches in only about one quarter of patients, and has an equal gender distribution. In contrast to post-trauma and spontaneous dissections, endovascular procedure-related dissection is generally diagnosed immediately during the procedure that caused the dissection. About a third of the recognized instrumentation-related renal artery dissections are asymptomatic, and pain and hypertension occur in about 50% of the patients, which is a much lower rate than is seen in primary dissections. Hematuria occurs in about 20% of patients.
In the half of reported cases of dissection following renal artery intervention, the patients underwent renal artery reconstruction, usually involving a renal artery bypass. This approach is most successful when the dissection is confined to the main renal artery, when renal infarction has not yet occurred, and when revascularization can be accomplished quickly. Studies report patients treated with endoluminal techniques, which have the advantage of immediacy. Successful endoluminal treatment usually requires placing a stent or covered stent to obliterate the false lumen and reexpand the true lumen. In some cases, adjunctive thrombolysis is needed, particularly if partial or distal thrombosis or embolization has occurred. The key to successful endoluminal treatment is the ability to maintain access to the true lumen distal to the dissection and lack of involvement of segmental branches. Occasionally, particularly when dissection extends distally beyond what could feasibly be treated either with stents or operative reconstruction, endoluminal distal fenestration of the septum provides the only option for improving distal perfusion.
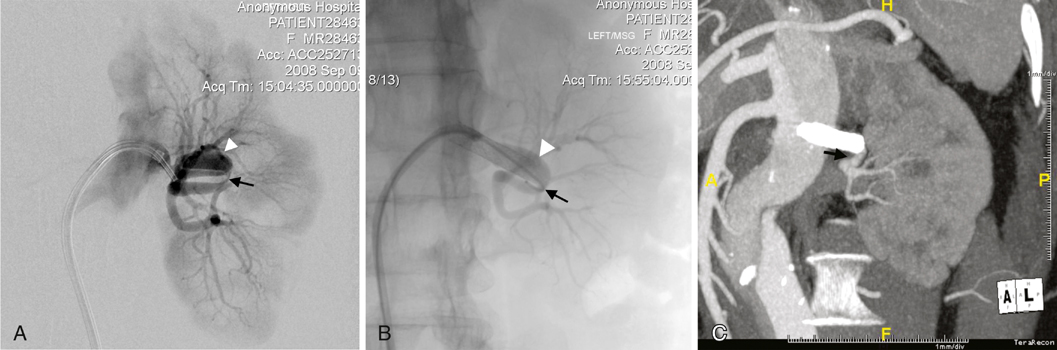
In early series, about one third of patients were observed, either because they were clinically stable (symptoms resolved, hypertension resolved or was medically controlled, and renal function remains normal or only minimally impaired) or because there were no evident treatment options. In this patient group, interval imaging often shows remodeling of the dissected artery, as well as some improvement in renal parenchymal perfusion (Figure 1), although complete normalization of arterial anatomy and renal perfusion is uncommon. Occasionally, improvement is unexpectedly dramatic. It is important to keep this in mind when determining how aggressive to be in attempting endoluminal treatment, which has the risk of worsening the dissection, or surgery, which has the risk of resulting in a nephrectomy. Although early series reported at least a 15% incidence of nephrectomy, it is important to avoid early nephrectomy whenever possible, because significant spontaneous improvement of the abnormal arterial anatomy and impaired renal perfusion can occur. Nephrectomy should be reserved for patients who develop refractory hypertension or other symptoms that suggest clinical instability.