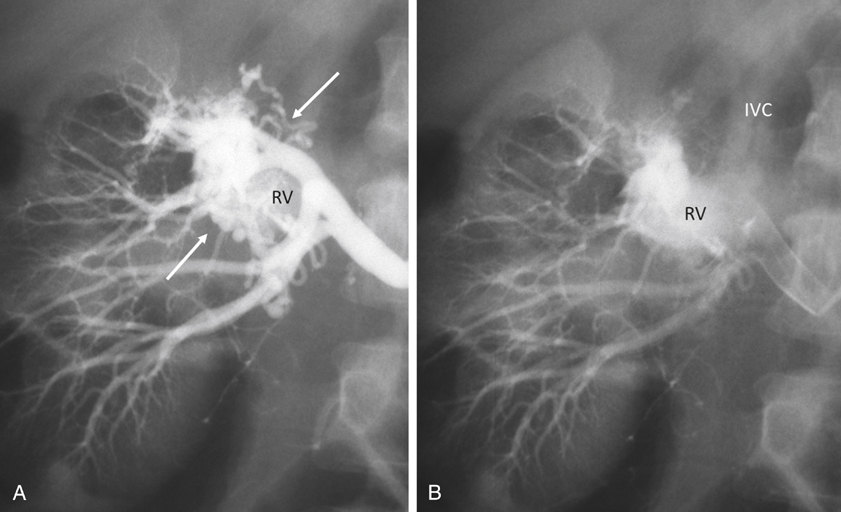
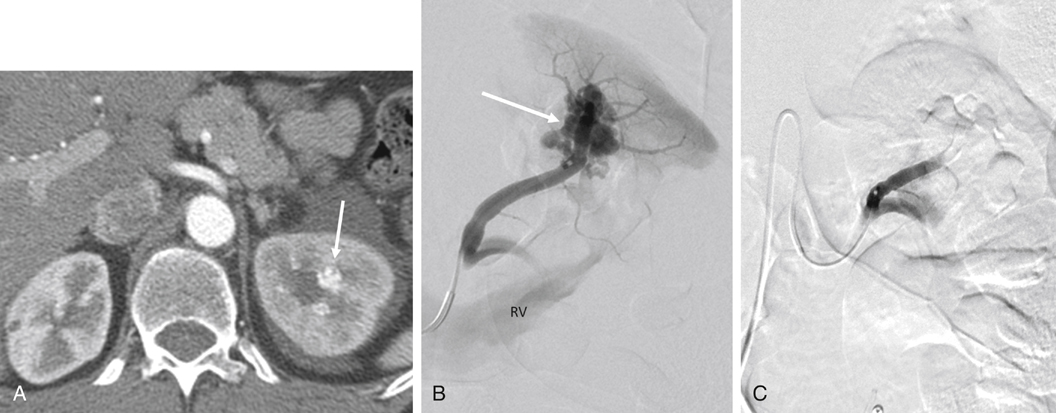
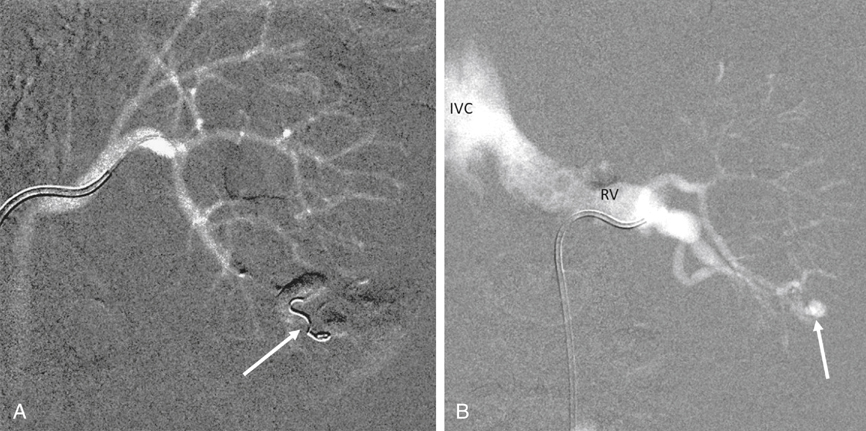
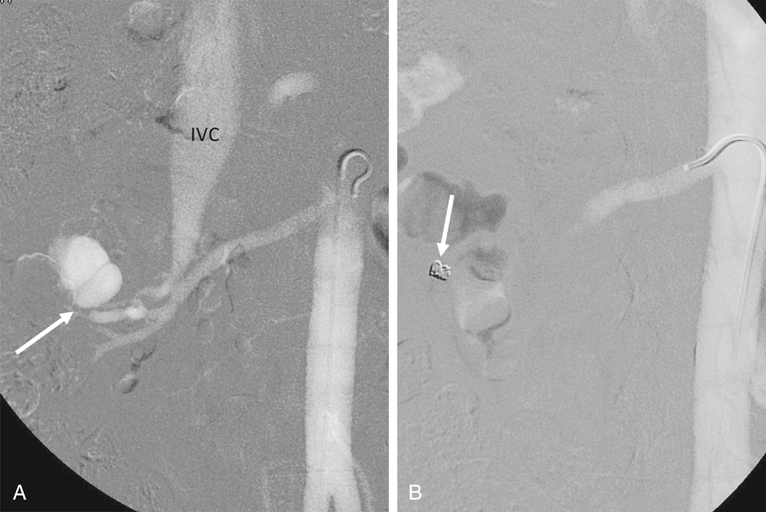
AVMs are congenital, are characterized by an arteriovenous communication at the arteriole and venule level, and are usually supplied by multiple feeding arteries (Figures 1 and 2). These malformations are either cirsoid, with multiple arteriovenous communications, or cavernous, with well-defined arterial and venous channels. In contrast, the acquired AVF usually has a single feeding artery supplying a direct communication between an artery and a vein (Figure 3). Arteriographic study should begin with an anteroposterior aortogram. The frame rates for the aortogram should cover the arterial phase at 3 to 4 frames per second. A flush catheter (5-Fr pigtail or 5-Fr Omni flush) should be placed so that the side holes are at the level of the renal arteries, and contrast medium should be injected at 15 to 20 mL/sec for 2 seconds. Carbon dioxide gas (CO2) can be used as an alternative contrast agent in the patient with contrast medium allergy, with kidney failure, or at high risk for contrast-induced nephropathy. Because of the low viscosity, CO2 is more sensitive in filling the AV fistula (see Figure 3). The angiographic abnormalities of renal AVMs and AVFs depend on the etiology, size, location, and extent of the lesions. Small, localized congenital AVMs have dilated, tortuous, and coiled vascular channels grouped in a cluster, which are supplied by multiple dilated branches from the segmental or interlobar arteries. The lesion is usually located in the hilar or pericalyceal region, ranging in size from 3 to 4 cm. They can occur in the upper polar, interpolar, or lower polar regions. Arteriovenous shunting, although often minimal, is always present and is demonstrated by early and dense filling of the vein. Like AVMs that occur elsewhere, neither capillary nor parenchymal contrast accumulation is present during the nephrographic phase (see Figure 1). Small polypoid defects of the calyces may be present. Traumatic AVFs are usually solitary, with an easily identified feeding artery and draining vein (see Figure 3). These may be located in the renal medulla or parenchyma and can involve the segmental, interlobar, or arcuate arteries. Pseudoaneurysms occasionally coexist with traumatic fistulas (Figures 3 and 4). Spontaneous AVFs can occur in patients with renal artery aneurysms and renal artery fibrodysplasia. Renal angiography demonstrates any large aneurysm associated with an AVF. In such cases, the feeding artery and the draining vein are usually dilated. Arteriovenous shunting associated with arterial fibrodysplasia has been shown to be minimal and small in size.
Renal Arteriovenous Malformations And Arteriovenous Fistulas
Classification
Angiographic Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
