The gradual loss of ventricular preexcitation during exercise stress testing (EST) has an unclear risk of an association with life-threatening arrhythmia and could be related to the accessory pathway (AP) location. We compared the loss of preexcitation during EST with the risk assessment during invasive electrophysiology testing and determined whether the loss of preexcitation correlates with the AP location. We retrospectively reviewed patients aged ≤21 years with ventricular preexcitation who had undergone both EST and an electrophysiology study. The patients were divided into 3 groups: sudden loss (SL), gradual loss (GL), or no loss (NL) of preexcitation during EST. A total of 76 patients were included, with 11 (14%) in the SL group, 18 (24%) in the GL group, and 47 (62%) in the NL group. The SL group demonstrated a longer cycle length with 1-to-1 conduction by way of the AP during incremental atrial pacing compared with the NL group (375 ± 135 ms vs 296 ± 52 ms, p = 0.002), with no difference between the GL and NL groups (325 ± 96 vs 296 ± 52 ms, p = NS). Of the patients with 1-to-1 AP conduction of <270 ms, none (0 of 11) were in the SL group compared to 18 of 47 in the NL group (p = 0.0017), with no significant difference in the GL group (5 of 18) compared to the NL group (p = NS). The patients in the GL group were more likely to have a left-sided AP (14 of 18) than the NL group (17 of 47, p = 0.002) and the SL group (3 of 11, p = 0.002). In conclusion, a sudden loss of preexcitation during an EST predicted a long cycle length with 1-to-1 conduction by way of the AP. Also, the AP conduction characteristics in patients with GL compared to those with NL did not differ, and the GL of preexcitation was more frequently seen in patients with a left-sided AP.
The loss of ventricular preexcitation during exercise stress testing (EST) in patients with Wolff-Parkinson-White syndrome has been used to risk stratify those who might develop atrial fibrillation with rapid ventricular conduction and sudden death. The sudden loss of ventricular preexcitation during EST suggests a low-risk accessory pathway (AP). However, the gradual loss (GL) of ventricular preexcitation during EST has an unclear risk of rapid AP conduction and could be related to the AP location. We hypothesize that the GL of ventricular preexcitation represents preferential conduction through the atrioventricular (AV) node instead of the AP, particularly in left-sided APs in which fusion is more evident. We compared the type of loss of ventricular preexcitation during EST with the risk assessment during invasive electrophysiology studies and determined whether the loss of preexcitation correlates with the AP location.
Methods
After approval from our institutional review board, we performed a retrospective chart review of the electrophysiology database. Our study inclusion criteria were age ≤21 years, a diagnosis of ventricular preexcitation on the surface electrocardiogram, normal cardiac anatomy determined by echocardiogram, and EST and electrophysiology study from 2004 to 2010 at our institution. The demographic data, including age, weight, height, gender, medications, and anesthesia type used during the electrophysiology study, were recorded.
EST was performed using a treadmill and the standard Bruce protocol, with a continuous 12-lead surface electrocardiogram and intermittent blood pressure monitoring.
The EST surface electrocardiographic tracings were reviewed for evidence of ventricular preexcitation at baseline and throughout the stress test. The type of loss of preexcitation that occurred during EST was noted. The subjects were divided into 3 groups: a sudden loss (SL) of ventricular preexcitation, a GL of preexcitation, and no loss (NL) of preexcitation. We defined a SL of preexcitation as a sudden beat-to-beat loss of preexcitation on the surface electrocardiogram during the stress test ( Figure 1 ) . GL was considered present in subjects with baseline ventricular preexcitation with either no preexcitation or an indeterminate presence of preexcitation at the peak heart rate and no clear beat-to-beat loss of preexcitation throughout the stress test. The subjects with no loss had ventricular preexcitation throughout the stress test. Three separate observers, two electrophysiologists, and a senior cardiology fellow, who were unaware of the patient groups, analyzed the surface electrocardiographic tracings. If the reviewers disagreed regarding the group placement, the electrocardiograms were reviewed to arrive at a consensus.
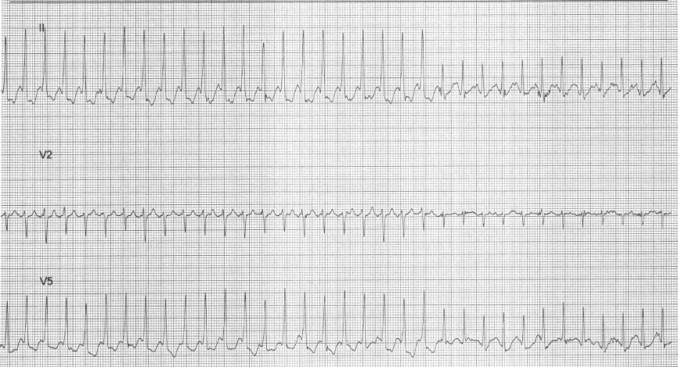
Electrophysiology studies were performed with the patients under general anesthesia or conscious sedation. Four catheters were positioned in the right atrial appendage, right ventricular apex, coronary sinus, and the His bundle position. Baseline intracardiac intervals, incremental atrial pacing, programmed atrial extrastimulus testing, incremental ventricular pacing, and ventricular extrastimulus testing were performed. The shortest cycle length with 1-to-1 conduction by way of the AP during incremental atrial pacing, the AP effective refractory period, and the shortest ventricular preexcited RR interval during atrial fibrillation both with and without isoproterenol were measured for risk assessment. Endocardial mapping and/or ablation with either a radiofrequency catheter (EZ Steer, Biosense Webster, Diamond Bar, California) or cryocatheter (Freezor 4 mm or Freezor Xtra 6 mm, Medtronic CryoCath Technologies, Montreal, Quebec, Canada) were used to determine the AP location. Conduction by way of the AP during incremental atrial pacing without isoproterenol was used as the main measure of risk assessment because atrial fibrillation was not induced in all patients.
The Student t test was used to compare continuous variables, and the chi-square test for categorical variables. p Values <0.05 were considered statistically significant.
Results
A total of 76 patients met the inclusion criteria, 45 (59%) were male. The median age of the group was 15.1 years (range 6.0 to 20.8) and the median weight was 58.2 kg (range 31.8 to 100). No difference was found in the demographic information among the groups, except the NL group was younger than the GL group ( Table 1 ). Of the 76 APs, 34 (45%) were left sided, 24 (32%) were septal, and 18 (23%) were right sided. All the APs were atrioventricular pathways.
| Group | p Value | ||||
|---|---|---|---|---|---|
| SL | GL | NL | SL vs NL | GL vs NL | |
| Patients (n) | 11 | 18 | 47 | ||
| Age (years) | NS | 0.02 | |||
| Median | 15.6 | 15.6 | 14.2 | ||
| Range | 9.6–18 | 9.6–20.8 | 6–19.9 | ||
| Male | 5 (45%) | 10 (56%) | 30 (64%) | NS | NS |
| Weight (kg) | NS | NS | |||
| Median | 53.2 | 59.9 | 58.6 | ||
| Range | 38.6–80 | 33.2–86 | 31.8–100 | ||
| Height (cm) | NS | NS | |||
| Median | 163 | 162.5 | 162.5 | ||
| Range | 145–183 | 144–184 | 131–190 | ||
| Antiarrhythmic medications | 3 (27%) | 2 (11%) | 5 (11%) | NS | NS |
| Anesthesia nongeneral | 0 | 1 (6%) | 1 (2%) | NS | NS |
| Atrial fibrillation induced | 7 (64%) | 13 (72%) | 23 (49%) | NS | NS |
| One-to-one conduction by accessory pathway on electrophysiology study (ms) | 375 ± 135 | 325 ± 96 | 296 ± 52 | 0.002 | NS |
| Left-sided accessory pathway | 3 (27%) | 14 (78%) | 17 (36%) | NS | 0.002 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


