Failure to intensify medication and failure to adhere to medication have been shown to contribute to suboptimal low-density lipoprotein cholesterol goal attainment. To examine whether nonadherence to statins in 126,903 patients on stable statin therapy is associated with subsequent treatment intensification, we conducted a retrospective analysis using an integrated pharmacy and medical claims database. Pharmacy claims were analyzed to determine whether nonadherence, as measured by proportion of days covered on statins <80%, was associated with intensification of statin treatment over a 360-day follow-up. Of 11,361 patients who had treatment intensification, 44% were previously nonadherent to statins. Patients whose treatment was intensified had slightly lower adherence to statin therapy than those without intensification (76% vs 78%, p <0.0001) and were more likely to be nonadherent as defined by proportion of days covered <80% (44% vs 37%, p <0.0001). After controlling for confounding factors, patients nonadherent to statins were 30% more likely to have treatment intensification compared to adherent patients (odds ratio 1.30, 95% confidence interval 1.25 to 1.36). In addition, patients with statin intensification were more likely to be younger, women, and have coronary artery disease, diabetes, hypertension, dyslipidemia, stroke, peripheral arterial disease, heart failure, or depression. Primary care physicians were more likely to escalate therapy than cardiologists. In conclusion, nearly 1/2 of patients with therapy escalation were nonadherent to statins. Clinicians should inquire about adherence and consider adherence before escalating statin therapy.
Statins are considered the preferred pharmacologic choice for patients in whom lifestyle modifications fail to achieve low-density lipoprotein (LDL) cholesterol targets. In the real world many patients do not reach their LDL cholesterol goals despite treatment with statins. Failure to intensify medications and failure to adhere to medications have been shown to contribute to suboptimal LDL cholesterol goal attainment. The objective of this study was to determine whether nonadherence to statins is associated with subsequent treatment intensification of lipid therapy in patients who were previously on a stable dose of statin therapy.
Methods
We analyzed de-identified medical and pharmacy claims data from the Medco National Integrated Database, which contains 39 months of medical and pharmacy claims data for approximately 13 million patients enrolled in 450 different health plans including fee-for-service, preferred provider, and managed care plans sponsored by insurance companies, employers, and government organizations. The medical claims contain all inpatient, outpatient, nursing home, laboratory, and diagnostic test claims received by the insurance plan. Laboratory values and medical care paid out of pocket or through Medicare are not included in the database.
We evaluated patients 18 to 62 years old as of January 1, 2009 who were continuously enrolled in the health plan for ≥27 months from April 1, 2008 through December 31, 2010. Patients who received a stable dose of a statin for ≥180 days from January 1, 2009 through December 31, 2009 were included in the analysis. The first possible start date of the stable period was January 1, 2009, and the index date was defined as the first day on which the patient had been on stable statin therapy for 180 days. The follow-up period was defined as 360 days after the index date and was used to identify subsequent treatment intensification. The baseline year was 360 days before the treatment intensification date. For patients without treatment intensification, the baseline year was the same as the follow-up period. The baseline year was used to calculate statin adherence and patient and physician characteristics ( Figure 1 ).
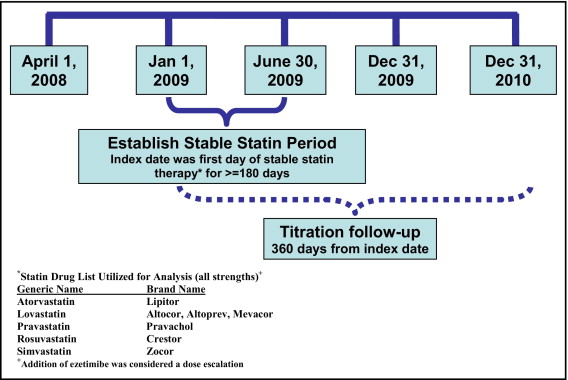
The statin dose was considered stable if the patient had ≥2 claims for the same statin drug and dose or an equivalent dose of a different statin during a 180-day period, and the patient could not have any claims for ezetimibe during the stable period. Patients selected were on statin therapy for ≥12 months before the index date and had ≥1 additional statin claim during the follow-up period. Treatment intensification was defined as a prescription for a statin with an increased daily dose equivalent or the addition of ezetimibe during the 360 days after the statin index date. Statin dose equivalency was based on expected LDL cholesterol decrease for each dose of an individual statin ( online Appendix A ). Patients were excluded if they were >62 years because of the possibility of incomplete medical data with dual Medicare coverage or had a history of malignant neoplasms other than nonmelanoma skin cancers, which were identified using International Classification of Diseases, Ninth Revision, Clinical Modification codes (140* to 172*, 174* to 209*).
Medication adherence was calculated using proportion of days covered (PDC) based on the total number of days with statins on hand as determined by fill dates and days’ supply in pharmacy records during the baseline year (360 days). PDC is a commonly used measurement of medication adherence based on pharmacy data, and patients with PDC ≥80% were considered adherent to therapy.
Key co-morbidities were identified based on the presence of International Classification of Diseases, Ninth Revision, Clinical Modification codes in medical claims during the baseline period for coronary artery disease (CAD; 410*, 411*, 412*, 413*, 414*, 996.03), diabetes mellitus (249* and 250*), hypertension (401* to 405*), dyslipidemia (272*), stroke (430* to 438*, 997.02), peripheral arterial disease (443.9), heart failure (402.11, 402.91, 404.11, 404.13, 404.91, 404.93, 428*), depression (296.2*, 296.3*, 298.0, 300.4, 309, 309.1, 309.28, 311), and end-stage renal disease (403*, 404*, 585.3 to 585.9, 586).
Bivariate associations were examined using unpaired t tests for continuous variables and chi-square tests for categorical variables. Multivariate logistic regression was used to evaluate independent associations between statin nonadherence and subsequent treatment intensification, adjusting for co-morbidities, age, gender, statin PDC, prescriber specialty of the first statin claim during the stable period, and pharmacy channel (mail or retail). Regression analysis was conducted using PDC as a bivariate measurement (adherent vs nonadherent). All analyses were performed with SAS 9.1 (SAS Institute, Cary, North Carolina).
Results
The study population was comprised of 126,903 patients who received ≥6 months of a stable statin regimen and remained eligible for 12 months of follow-up ( Figure 1 ). Of these patients, 11,361 had dose intensification during the follow-up year. Patients whose treatment was intensified had slightly lower mean statin PDC than those without a dose intensification (76% vs 78%, p <0.0001), and they were more likely to be nonadherent to statin therapy as defined by PDC <80% (44% vs 37%, p <0.0001; Table 1 ). Incidence of dose intensification by baseline adherence level is presented in Table 2 .
| Variable | All Patients (n = 126,903) | Dose Escalation | p Value | |
|---|---|---|---|---|
| With (n = 11,361) | Without (n = 115,542) | |||
| Age (years), mean ± SD | 54.2 ± 6.5 | 54.0 ± 6.6 | 54.3 ± 6.5 | <0.0001 |
| Women | 42.1% | 43.2% | 42.0% | 0.014 |
| Mean statin proportion of days covered (%) | 78% | 76% | 78% | <0.0001 |
| Patients with proportion of days covered ≥80% (adherent) | 62.4% | 55.5% | 63.1% | <0.0001 |
| Hypertension | 52.2% | 56.3% | 51.8% | <0.0001 |
| Coronary artery disease | 11.2% | 16.5% | 10.7% | <0.001 |
| Diabetes mellitus | 26.8% | 29.3% | 26.5% | <0.001 |
| Stroke | 3.2% | 5.2% | 3.0% | <0.0001 |
| Peripheral artery disease | 1.2% | 1.7% | 1.1% | <0.0001 |
| Heart failure | 1.6% | 2.3% | 1.5% | <0.0001 |
| Depression | 8.0% | 9.4% | 7.9% | <0.0001 |
| End-stage renal disease | 2.2% | 2.5% | 2.1% | 0.013 |
| Dyslipidemia | 74.5% | 83.4% | 73.6% | <0.0001 |
| Charlson score, mean ± SD | 0.59 ± 0.95 | 0.69 ± 1.02 | 0.58 ± 0.94 | <0.0001 |
| Medications in baseline year | ||||
| Fibrates | 7.2% | 8.7% | 7.1% | <0.0001 |
| Statin combination | 0.4% | 0.5% | 0.3% | <0.001 |
| Nicotinic acid (niacin) | 3.7% | 4.1% | 3.6% | 0.024 |
| Bile acid sequestrant | 0.6% | 0.6% | 0.6% | NS |
| Ezetimibe | 0.1% | 0.8% | 0.0% | <0.0001 |
| Number of medications on index date, mean ± SD | 3.5 ± 2.6 | 3.5 ± 2.7 | 3.5 ± 2.6 | NS |
| PDC Over 12 Months | |||||
|---|---|---|---|---|---|
| 1%–19% | 20%–39% | 40%–59% | 60%–79% | 80%–100% | |
| All patients | 2,767 (2.2%) | 6,845 (5.4%) | 13,893 (10.9%) | 24,225 (19.1%) | 79,173 (62.4%) |
| Men | 1,431 (51.1%) | 3,726 (51.0%) | 7,784 (55.1%) | 13,938 (56.2%) | 46,631 (58.3%) |
| Women | 1,336 (48.9%) | 3,119 (49.0%) | 6,109 (44.9%) | 10,287 (43.8%) | 32,542 (41.7%) |
| Age 18–44 years | 458 (19.7%) | 956 (12.9%) | 1,636 (13.6%) | 2,591 (9.8%) | 5,622 (7.4%) |
| Age 45–62 years | 2,309 (80.3%) | 5,889 (87.1%) | 12,257 (86.4%) | 21,634 (90.2%) | 73,551 (92.6%) |
| Patients with dose escalation | 223 (2.0%) | 627 (5.5%) | 1,541 (13.6%) | 2,664 (23.4%) | 6,306 (55.5%) |
The fully adjusted logistic regression model ( Table 3 ) revealed that treatment intensification was 30% more likely (odds ratio 1.30) in nonadherent patients. When we analyzed statin PDC as a continuous variable, it was associated with an odds ratio of 0.996; therefore, each percent point increase in PDC was associated with a 0.4% lower likelihood of dose escalation.
| Covariate | Minimally Adjusted Model ⁎ | Fully Adjusted Model † | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age | — | — | 0.99 | (0.99 – 0.99) |
| Gender (control male gender) | — | — | 1.08 | (1.03–1.12) |
| Statin proportion of days <80% (bivariate) | 1.36 | (1.30–1.41) | 1.30 | (1.25–1.36) |
| Statin proportion of days (continuous variable) ‡ | 0.995 | (0.994–0.996) | 0.996 | (0.996–0.997) |
| Hypertension | 1.05 | (1.01–1.09) | 1.05 | (1.01–1.10) |
| Dyslipidemia | 1.73 | (1.65–1.83) | 1.75 | (1.66–1.84) |
| Coronary artery disease | 1.58 | (1.49–1.67) | 1.68 | (1.58–1.78) |
| Diabetes mellitus | 1.09 | (1.04–1.14) | 1.07 | (1.03–1.12) |
| Stroke | 1.52 | (1.39–1.67) | 1.53 | (1.39–1.68) |
| Peripheral artery disease | 1.19 | (1.02–1.39) | 1.19 | (1.02–1.39) |
| Heart failure | 1.16 | (1.01–1.33) | 1.14 | (1.00–1.31) |
| Depression | 1.15 | (1.07–1.23) | 1.14 | (1.06–1.22) |
| End-stage renal disease | 1.00 | (0.88–1.14) | 0.97 | (0.85–1.10) |
| Prescriber of first stable statin claim (control primary care provider) | ||||
| Cardiologist | 1.09 | (1.01–1.17) | 0.84 | (0.77–0.91) |
| Endocrinologist | 1.09 | (0.95–1.25) | 1.07 | (0.93–1.23) |
| Other | 1.15 | (1.09–1.20) | 1.09 | (1.04–1.14) |
| Source of prescriptions (control retail only) | ||||
| Mail only | 1.50 | (1.44–1.56) | 1.43 | (1.37–1.49) |
| Mail and retail | 1.06 | (0.99–1.15) | 1.01 | (0.94–1.09) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


