Atrial arrhythmia (AA) is common in adult patients with congenital heart disease (CHD). To enable the prevention of AA or its complications, timely identification of adult patients with CHD at risk of AA is crucial. Long total atrial activation times have been related to AA. Tissue Doppler imaging (TDI) permits noninvasive evaluation of the total atrial conduction time (PA-TDI duration). The present study evaluated the association between the PA-TDI duration and the development of AA in adult patients with CHD. A total of 223 adult patients with CHD were followed up for the occurrence of AA after PA-TDI duration assessment. The PA-TDI duration was defined as the interval from the onset of the P wave on the electrocardiogram to the peak of the A′ wave at the lateral atrial wall on TDI tracings. Among the various clinical and echocardiographic parameters, the association between the PA-TDI duration and AA occurrence was investigated. The median follow-up was 39 months (interquartile range 21 to 57). A PA-TDI duration of ≥126 ms was associated with AA during follow-up (log-rank, p <0.001). On multivariate analysis, a PA-TDI duration >126 ms (hazard ratio 2.25, 95% confidence interval 1.21 to 4.19) and history of AA (hazard ratio 4.89, 95% confidence interval 2.75 to 8.71) were independently associated with the occurrence of AA. In conclusion, PA-TDI duration and a history of AA were independently associated with the occurrence of AA in adult patients with CHD. The PA-TDI duration is a useful tool to identify patients with CHD at risk of AA during follow-up.
In patients with congenital heart disease (CHD), longstanding altered atrial hemodynamics, increased atrial pressure, and surgical atrial scarring promote atrial remodeling and electrical conduction abnormalities. These electrical disturbances include prolonged atrial conduction time, favoring the onset of atrial arrhythmia (AA). Recently, a novel echocardiographic tool using tissue Doppler imaging (TDI), has been shown to be a fast, easy, and reliable method to assess the total atrial conduction time. With color-coded TDI of the atria, the interval from the onset of the P wave on the electrocardiogram to the peak of the A′ wave on the TDI tracings of the lateral atrial wall can be measured (so-called PA-TDI duration). The PA-TDI duration reflects the total atrial conduction time and has been shown to be associated with AA in patients with cardiac disease. However, the association between the PA-TDI duration and the occurrence of AA in patients with CHD has not been evaluated. The present study investigated the association between the PA-TDI duration and the occurrence of AA in adult patients with CHD. Additionally, this association was studied further in the subsets of adult patients with “severe CHD” and “nonsevere CHD.”
Methods
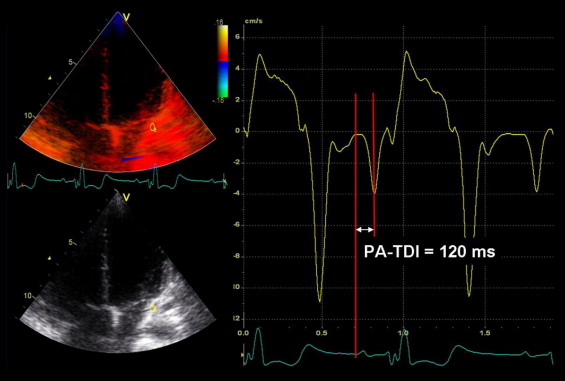
Adult patients with CHD who were followed up for the occurrence of AA from the first available TDI echocardiogram in sinus rhythm (baseline) until November 2010 were included. The patients without sinus rhythm at baseline or without additional clinical follow-up visits were excluded from the analysis. AA was defined as atrial tachycardia, atrial flutter, or atrial fibrillation on the surface electrocardiogram (ECG), 24- or 48-hour Holter ECG, or implantable cardioverter-defibrillator device recordings. The clinical and echocardiographic data were retrieved from the departmental cardiology information system (EPD-Vision, Leiden University Medical Center, Leiden, The Netherlands) and the echocardiographic database, respectively. All adult patients with CHD underwent a complete clinical evaluation at baseline. The CHD diagnosis was specified as “severe CHD” or “nonsevere CHD,” as described previously. In addition, the use of β blockers, antiarrhythmic medication, or angiotensin-converting enzyme inhibitors at baseline was recorded. Furthermore, the presence of hypertension at baseline (systolic pressure >140 mm Hg and/or diastolic pressure >90 mm Hg) and any surgical procedures were documented. In addition, the baseline echocardiographic variables included the dimensions and systolic function of the systemic ventricle and the total atrial conduction time as measured with TDI (PA-TDI). Transthoracic echocardiographic images were acquired using a commercially available system equipped with a 3.5-MHz transducer (Vivid-7, GE Vingmed Ultrasound AS, Horten, Norway). The subjects were placed in the left lateral decubitus position during image acquisition. Standard 2-dimensional images were acquired from the parasternal (long- and short-axis) and apical (2- and 4-chamber and long-axis) views and digitally stored in cine-loop format. The analyses were performed off-line using EchoPac, version 108.1.5 (General Electric Medical Systems, Horten, Norway). The end-diastolic and end-systolic volumes of the systemic ventricle were measured in the apical 2- and 4-chamber views, and the ejection fraction was calculated. Furthermore, TDI scans from the apical 4-chamber view were obtained. The frame rate was ≥120 frames/s, and ≥3 consecutive beats were recorded. The PA-TDI duration was determined as shown in Figure 1 . The patients were followed up at 6- to 12-month intervals for the occurrence of AA. Of the several clinical and echocardiographic parameters, the independent determinants of AA occurrence during follow-up were identified. Subsequently, the association between the PA-TDI duration and the occurrence of AA in adult patients with CHD was assessed. Atrial arrhythmia was determined on the basis of the electrocardiographic findings consistent with the diagnosis of atrial flutter, atrial tachycardia, or atrial fibrillation on the 12-lead electrocardiograms and 24-hour electrocardiographic Holter recordings. In patients with an implantable cardioverter-defibrillator, stored electrocardiographic information was interrogated every 6 months, and episodes of AA were recorded.
The distribution of continuous variables was tested by the Kolmogorov-Smirnov test. Continuous variables with a normal distribution are expressed as the mean ± SD. Continuous variables with no normal distribution are expressed as the median and interquartile range. Categorical variables are presented as numbers and percentages. Differences between the patients with and without AA during follow-up were analyzed using the 2-sided unpaired Student’s t test or Mann-Whitney U test, as appropriate, for continuous data and the chi-square test for categorical data. The study population was divided according to the optimal PA-TDI duration cutoff value to be associated with AA occurrence. The optimal PA-TDI cutoff value with maximized sensitivity and specificity was determined with receiver operating characteristic curve analysis. Cumulative event rates were calculated using the Kaplan-Meier survival analysis, and the time-to-event data with respect to the occurrence of AA were compared with the log-rank test between the 2 groups of patients, dichotomized by the PA-TDI cutoff value. In addition, univariate and multivariate Cox proportional hazards regression analyses were performed to identify the clinical and echocardiographic parameters associated with the occurrence of AA. Only significant univariate parameters were entered as variables in the multivariate analysis using the enter method. Hazard ratios and 95% confidence intervals were calculated for each independent variable. The data were analyzed using SPSS, version 17.0, software (SPSS, Chicago, Illinois). A p value of <0.05 was considered statistically significant.
Results
A total of 223 adult patients with CHD were included (mean age 49 ± 12 years, 49% men). During a median follow-up duration of 39 months (interquartile range 21 to 57), 57 patients (26%) presented with AA. The clinical and echocardiographic characteristics of both groups of adult patients with CHD who presented with AA during follow-up versus patients without AA are listed in Table 1 . Based on the receiver operating characteristic curve analysis, a cutoff value of PA-TDI of 126 ms permitted the identification of patients with CHD who presented with AA during follow-up, with a sensitivity of 80% and specificity of 61% (area under the curve 0.79). Subsequently, this cutoff value for the PA-TDI duration was used to dichotomize the population, and the time-to-event data with respect to the occurrence of AA was evaluated with Kaplan-Meier survival analysis. The cumulative event rate in the group with a PA-TDI duration of ≥126 ms was 20%, 26%, 36%, and 43% at 12, 24, 36, and 48 months, respectively. In contrast, in the group of patients with a PA-TDI duration of <126 ms, the corresponding event rates were 5%, 6%, 8%, and 8% (chi-square 21.10, log-rank p <0.001; Figure 2 ) . In addition, the adult CHD population was divided into patients with “nonsevere CHD” and “severe CHD.” The receiver operating characteristic curve analysis showed that in patients with nonsevere CHD, a PA-TDI of ≥123.5 ms was associated with the occurrence of AA during follow-up (sensitivity 70%, specificity 60%, area under the curve 0.72), and a PA-TDI of ≥129.5 ms was associated with AA occurrence in the group of patients with “severe CHD” (sensitivity 80%, specificity 65%, area under the curve 0.80). These cutoff values were subsequently used to predict the time-to-occurrence of AA with Kaplan-Meier survival analysis in both subgroups. In the “nonsevere CHD” group, Kaplan-Meier survival analysis showed greater cumulative event rates in patients with a PA-TDI duration of ≥123.5 ms (chi-square 6.22, log-rank p = 0.012. The cumulative event rate in the “nonsevere CHD” group with a PA-TDI duration of ≥123.5 ms was 15%, 15%, 24%, and 27% at 12, 24, 36, and 48 months of follow-up, respectively. Likewise, in the group of patients with “severe CHD,” the cutoff value of a PA-TDI duration of ≥129.5 ms significantly predicted a greater cumulative rate of AA occurrence during follow-up (chi-square 17.34, log-rank p <0.001). The cumulative event rate in the patients with “severe CHD” with a PA-TDI duration of ≥129.5 ms was 28%, 42%, 47%, and 53% at 12, 24, 36, and 48 months of follow-up, respectively.
| Clinical and Echocardiographic Variables | Atrial Arrhythmia | p Value | |
|---|---|---|---|
| Yes (n = 57) | No (n = 166) | ||
| Age (years) | 51 ± 13 | 48 ± 12 | 0.245 |
| Gender | 0.511 | ||
| Male | 30 (53%) | 79 (48% | |
| Female | 27 (47%) | 87 (52%) | |
| Severe congenital heart disease ⁎ | 30 (53%) | 57 (34%) | 0.015 |
| Previous history of atrial arrhythmia | 35 (61%) | 26 (16%) | <0.001 |
| Medication | |||
| β Blockers | 31 (54%) | 25 (15%) | <0.001 |
| Antiarrhythmic agents | 5 (9%) | 10 (6%) | 0.475 |
| Angiotensin-converting enzyme inhibitors | 21 (36%) | 32 (19%) | 0.007 |
| Hypertension | 5 (9%) | 23 (14%) | 0.318 |
| Surgical procedure during follow-up | 14 (25%) | 15 (9%) | 0.003 |
| Systemic ventricle end-diastolic volume (ml) | 124 ± 46 | 118 ± 49 | 0.441 |
| Systemic ventricle end-systolic volume (ml) | 0.216 | ||
| Median | 64 | 58 | |
| Range | 46–84 | 42–73 | |
| Systemic ventricle ejection fraction (%) | 46 ± 13 | 48 ± 11 | 0.118 |
| Tissue Doppler imaging-derived total atrial conduction time (ms) | 152 ± 33 | 121 ± 24 | <0.001 |
⁎ Severe congenital heart disease included atrioventricular septum defect, tetralogy of Fallot, D-transposition after arterial switch, D-transposition after atrial switch, L-transposition, truncus arteriosus, and tricuspid atresia; Nonsevere congenital heart disease included anomalous pulmonary venous return, aortic stenosis, ventricular septum defect, atrial septum defect, atrial septum defect, ventricular septum defect, coarctation of the aorta, Ebstein’s anomaly, patent ductus arteriosus, pulmonary stenosis, pulmonary stenosis and atrial septum defect, pulmonary stenosis, and ventricular septum defect.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


