This retrospective study assessed long-term clinical outcomes of patients with orthotopic heart transplantation (OHT) and transplant coronary artery disease (TCAD) who developed in-stent restenosis (ISR) after percutaneous coronary intervention (PCI). TCAD is a major cause of morbidity and mortality after the first year after OHT. Description of outcomes in patients with ISR after revascularization for TCAD is limited. One hundred five patients underwent PCI with bare-metal stents or drug-eluting stents at the UCLA Medical Center from 1995 throughout 2009, of whom 83 patients (79.0%) underwent repeat angiography for clinical symptoms or surveillance. The primary end point was the composite of death, myocardial infarction, or repeat OHT. ISR occurred in 26 patients (31.3%) who underwent follow-up angiography. Initial treatment strategies for the 26 patients with ISR were target vessel revascularization in 19 (73.1%), repeat OHT in 3 (11.5%), and medical therapy in only 4 (15.4%). At 7 years freedom from the primary end point was lower in patients with ISR compared to patients without ISR (27.9% vs 63.2%, p = 0.006, log-rank test) primarily driven by a lower survival rate in patients with ISR (38.5% vs 84.2%, p <0.001, log-rank test). Although numerically smaller in patients with ISR, there were no statistically significant differences in freedom from myocardial infarction (80.8% vs 91.2%, log-rank p = 0.18) and freedom from repeat OHT (73.1% vs 84%, p = 0.22, long-rank test). In conclusion, patients with OHT who develop ISR after PCI have poor long-term prognosis. Improvements in prevention and treatment of TCAD such as increased pharmacotherapy are needed.
Transplant coronary artery disease (TCAD) commonly occurs after orthotopic heart transplantation (OHT), occurring in 32% of patients at 5 years and 53% at 10 years, and is a major cause of death and allograft loss after the first year after OHT. TCAD is characterized by an aggressive form of diffuse arterial narrowing. Ideal management of TCAD is unknown because treatment options for TCAD are few. Current options are associated with poor long-term success and there is because of limited characterization of outcomes after treatment. Patients after OHT comprise 1 of the highest-risk groups who undergo percutaneous coronary intervention (PCI), with poor long-term angiographic and clinical outcomes. PCI plays a palliative role for treatment of TCAD. There are no published data or recommendations on ideal treatment for in-stent restenosis (ISR) in TCAD, and descriptions of outcomes after identification and treatment of ISR are sparse. We evaluated outcomes of patients with TCAD who developed ISR after stenting.
Methods
Data on patients with TCAD who underwent PCI with a bare-metal stent or drug-eluting stent from 1996 through 2009 at the UCLA Medical Center and had angiographic evidence of binary restenosis (>50% diameter restenosis on repeat coronary angiogram) were retrospectively analyzed. Data on the first 82 patients in this study have been previously described. Approval of the institutional review board was obtained to review the PCI database for this study.
Standard techniques for PCI were used. Choices of anticoagulation and type of stent used were at the discretion of the operator. From 1996 through April 2003, bare-metal stents were used. From May 2003 through November 2009, PCI with drug-eluting stents using sirolimus (Cypher, Cordis, Johnson and Johnson Corporation, Miami, Florida), paclitaxel (Taxus, Boston Scientific Corporation, Natick, Massachusetts), or everolimus (Xience, Abbott Vascular, Santa Clara, California) was the preferred strategy. All patients were treated with a thienopyridine (ticlopidine or clopidogrel) for a minimum of 1 month if PCI with bare-metal stents was used and ≥6 months if PCI with drug-eluting stents was used and aspirin indefinitely. Management of ISR was based on clinical judgment after discussion with the transplant cardiologist.
The primary end point of this analysis was the composite of death, myocardial infarction, or repeat OHT. Secondary clinical end points were death, myocardial infarction, and repeat OHT. Myocardial infarction was diagnosed according to the universal definition with the detection of an increase and/or decrease of cardiac enzymes with ≥1 value >99th percentile of the upper limit of normal with evidence of myocardial ischemia and ≥1 of the following: (1) ischemic symptoms, (2) electrocardiographic changes indicative of new ischemia (new ST-T–wave changes or new left bundle branch block), (3) development of pathologic Q waves on electrocardiogram, or (4) new loss of viable myocardium or new regional wall motion abnormality on imaging. The Academic Research Consortium definition for definite or probable stent thrombosis was used.
Patient data were collected from medical records or from telephone interviews with physicians who followed them at another institution and recorded into a dedicated database. If repeat PCI was performed, surveillance angiography was performed at ≤6 months if clinically indicated. Reasons for incomplete follow-up were premature death or patient refusal.
Quantitative coronary analysis was performed with an automated edge detection computer analysis system (GE CA1000 Stenosis Analysis Application, GE Healthcare, Piscataway, New Jersey).
Continuous variables are expressed as mean ± SD and were compared by Student’s t tests. Categorical variables are expressed as percentage and were compared by chi-square statistics or Fisher’s exact test, as indicated. Time-to-events curves were determined by the Kaplan–Meier method and comparisons were made using log-rank test. A multivariable Cox proportional hazard model was created using baseline clinical and angiographic characteristics and procedure-related variables to identify independent predictors of the primary end point. Variables inserted into the Cox regression analysis included age, male gender, diabetes mellitus, hypertension, treatment with statin, left ventricular ejection fraction, elective PCI, age of allograft, symptom when ISR was detected, intravascular ultrasound, intra-aortic balloon pump, glycoprotein IIb/IIIa inhibitor, rotational atherectomy, drug-eluting stent, mean stent diameter, and mean stent length. A p value <0.05 was considered statistically significant. Statistical analysis was performed with SAS 9.1 (SAS Institute, Cary, North Carolina).
Results
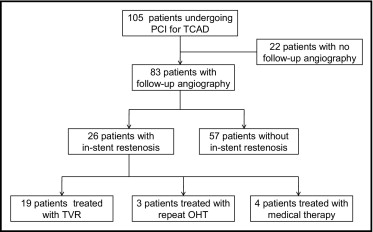
In total 105 patients underwent first-vessel PCI with bare-metal stents (n = 47) or drug-eluting stents (n = 58; Figure 1 ) . Of 83 patients (79%) who underwent follow-up angiography, ISR occurred in 26 patients (31%; Table 1 ). Median time to identification of ISR was 282 days. Mean angiographic stenosis for patients who developed ISR was 85.2 ± 11.8% ( Table 2 ).
| Variable | ISR | p Value | |
|---|---|---|---|
| Yes (n = 26) | No (n = 57) | ||
| Age (years) | 53.7 ± 14.9 | 56.9 ± 17.0 | 0.24 |
| Men | 65% | 77% | 0.26 |
| Diabetes mellitus | 31% | 28% | 0.44 |
| Hypertension | 69% | 63% | 0.59 |
| Treatment with statin | 73% | 63% | 0.38 |
| Left ventricular ejection fraction (%) | 48.8 ± 11.6 | 51.7 ± 11.3 | 0.98 |
| Elective percutaneous coronary intervention | 92% | 93% | 0.91 |
| Age of allograft (years) | 8.2 ± 4.2 | 8.9 ± 5.0 | 0.09 |
| Symptom when in-stent restenosis detected | |||
| Asymptomatic | 69% | N/A | |
| Angina/dyspnea on exertion | 19% | N/A | |
| Myocardial infarction | 8% | N/A | |
| Cardiac arrest | 4% | N/A | |
| Variable | ISR | p Value | |
|---|---|---|---|
| Yes | No | ||
| (n = 26) | (n = 57) | ||
| Percent angiographic stenosis | 85.2 ± 11.8 | N/A | |
| Coronary artery treated | 0.99 | ||
| Left main | 4% | 5% | |
| Left anterior descending | 46% | 47% | |
| Left circumflex | 19% | 19% | |
| Right | 31% | 28% | |
| Intravascular ultrasound | 35% | 35% | 0.97 |
| Intra-aortic balloon pump | 12% | 5% | 0.31 |
| Glycoprotein IIb/IIIa inhibitor | 31% | 21% | 0.34 |
| Rotational atherectomy | 4% | 2% | 0.56 |
| Drug-eluting stent | 46% | 54% | 0.15 |
| Mean stent diameter (mm) | 2.9 ± 0.4 | 3.0 ± 0.4 | 0.73 |
| Mean stent length (mm) | 22.9 ± 10.3 | 18.8 ± 10.1 | 0.28 |
In the 26 patients with ISR, initial treatment strategies were target vessel revascularization (TVR) in 19 (73.1%), repeat OHT in 3 (11.5%), and medical therapy only in 4 (15.4%). Of the 19 patients who underwent TVR, repeat stenting was performed in 16 (84.2%), brachytherapy in 1 (5.3%), balloon angioplasty in 1 (5.3%), and coronary artery bypass surgery in 1 (5.3%). Of the 11 patients who underwent further repeat angiography after TVR, 7 (63.6%) developed recurrent ISR.
Two patients who were initially treated with TVR died from stent thrombosis, although the relation to the restenotic lesion was not definitely ascertained. A 74-year-old man had ISR of a bare-metal stent placed in the right coronary artery and underwent TVR with 2 sirolimus-eluting stents and revascularization of the left anterior descending coronary artery with 2 sirolimus-eluting stents and left circumflex coronary artery with 1 sirolimus-eluting stent. The patient died suddenly 18 days later. A 60-year-old woman had ISR of a sirolimus-eluting stent in the left anterior descending coronary artery and subsequently underwent TVR. Four years later she died from autopsy-confirmed stent thrombosis of a paclitaxel-eluting stent 20 days after undergoing PCI of a de novo lesion in the right coronary artery.
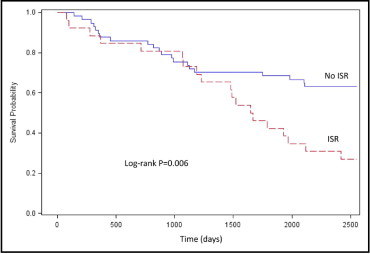
At 7 years, which was the mean duration of follow-up, freedom from the composite end point of death, myocardial infarction, or repeat OHT was lower in patients with ISR compared to patients without ISR (27.9% vs 63.2%, p = 0.006, log-rank test; Figure 2 ) primarily driven by a lower survival rate in patients with ISR (38.5% vs 84.2%, p <0.001, log-rank test; Figure 3 ) . Although numerically smaller in patients with ISR, there were no statistically significant differences between patients with ISR and patients without ISR in freedom from myocardial infarction (80.8% vs 91.2%, p = 0.18, log-rank test; Figure 4 ) and freedom from repeat OHT (73.1% vs 84%, p = 0.22, log-rank test; Figure 5 ) .