Decreased left ventricular (LV) longitudinal strain and increased circumferential LV strain have been demonstrated in patients with severe aortic stenosis (AS) and normal LV ejection fraction (LVEF). Biplane myocardial mechanics normalize after aortic valve replacement (AVR). This study objective was to examine LV mechanics before and soon after AVR in patients with AS and LV systolic dysfunction. Paired echocardiographic studies before and soon (7 ± 3 days) after AVR were analyzed in 64 patients with severe AS: 32 with normal LVEF (≥50%), 16 with mild to moderate LV dysfunction (LVEF <36% to 50%), and 16 with severe LV dysfunction (LVEF ≤35%). Longitudinal myocardial function was assessed from 3 apical views (average of 18 segments) and circumferential function was assessed at mid-LV and apical levels (average of 6 segments per view). Strain, strain rate, and mid-LV and apical rotations were measured using 2-dimensional velocity vector imaging. Before AVR (1) longitudinal strain was low in all patients and correlated with LVEF (ρ = 0.74, p <0.001), (2) mid-LV circumferential strain was supranormal in patients with normal LVEF and low in patients with low LVEF (ρ = 0.88, p <0.001), and (3) apical rotation was highest in patients with mild to moderate LV dysfunction. After AVR, LVEF increased in patients with LV dysfunction and myocardial mechanics partly normalized. In conclusion, compensatory mechanisms (high circumferential strain in patients with preserved LVEF and increased apical rotation in patients with mild to moderate LV dysfunction) were observed in patients with severe AS. Compensatory mechanics were lost in patients with severe LV dysfunction. AVR partly reversed these changes in patients with LV dysfunction.
We previously reported abnormal myocardial mechanics in patients with severe aortic stenosis (AS) with normal left ventricular (LV) ejection fraction (LVEF). Longitudinal strain was decreased but circumferential strain increased (perhaps as a compensatory mechanism) before aortic valve replacement (AVR). These abnormalities were reversed after AVR. Decreased longitudinal strain has been previously demonstrated in patients with AS, whereas circumferential strain has been low or increased. in different studies. Although the study definition of “normal” LVEF has been consistent, average and median values of LVEF have been quite different in patients included in various studies. This suggests that observed differences between studies might represent differences in baseline LV function, with different types and degree of benefit after AVR. The aims of our study were (1) to define biplane LV mechanics in patients with AS and normal or variably abnormal LV function and (2) to examine changes in LV mechanics after AVR.
Methods
Three hundred sixty-six patients underwent AVR for severe AS from January 2004 through September 2009 at the Rambam Health Care Campus (Haifa, Israel). Of these, 32 patients had (1) preoperative low LVEF (<50%), (2) available echocardiographic studies before and soon after AVR in digital imaging and communications in medicine format with a minimum 2-dimensional frame rate of 40 frames/s and good demarcation of the LV endocardial border, and (3) no other significant valvular heart disease. Patients with coronary artery disease or preoperative wall motion abnormalities were included. Patients were subgrouped according to preoperative LVEF: 16 patients with mild to moderate LV dysfunction (LVEF 36% to 49%, “low LVEF”) and 16 patients with severe LV dysfunction (LVEF ≤35%, “very low LVEF”). These patients were compared to 32 age- and gender-matched patients with severe AS and normal LVEF (≥50%, “normal LVEF”) who underwent AVR at our institution and who were included in our previous report. The study was approved by the hospital research ethics board.
Two-dimensional Doppler parameters were measured according to guidelines of the American Society of Echocardiography. LVEF was assessed using the biplane Simpson method. Aortic valve area was calculated by the continuity equation (before AVR) or estimated using normograms of valve prostheses (after AVR). LV subendocardial mechanics were analyzed using velocity vector imaging (Siemens Medical Systems, Mountain View, California), a 2-dimensional tissue-tracking software. Longitudinal strain and strain rate measurements were averaged from 18 segments from apical 2-, 3-, and 4-chamber views (6 segments in each standard view). Circumferential strain, strain rate, and rotation angles were averaged from 6 segments in each short-axis plane: midleft ventricle (papillary muscle) and apex. Averaged myocardial rotation angles were used to calculate apical to mid-LV twist, defined as maximal instantaneous apical to mid-rotation angle difference.
Off-line strain measurements were done by a single observer (S.C.). Twenty studies were reanalyzed by the same observer and another observer for assessment of intraobserver and interobserver variabilities.
Continuous data are reported as mean ± SD. Nonparametric tests were used. The 3 patient groups (normal LVEF, low LVEF, and very low LVEF) were compared using Wilcoxon rank-sum test and paired results before versus after AVR were compared using Wilcoxon signed-rank test. Linear correlations between strain components and standard echocardiographic Doppler parameters were assessed by Spearman correlation coefficient.
Results
Mean age of the study population was 71 ± 11 years, with no significant difference across subgroups ( Table 1 ). There were more men in the very-low–LVEF subgroup (not statistically significant). Although peak systolic transaortic pressure gradient was high in all patients, it was significantly lower in those with very low LVEF compared to the other subgroups. Aortic valve area was similar in all subgroups (0.8 ± 0.2 cm 2 in total study population). LV end-diastolic and end-systolic diameters and left atrial end-systolic diameter significantly increased with decreasing LVEF. Wall thickness was mildly increased and similar in all subgroups. Patients with normal LVEF and those with low LVEF had similar mitral inflow diastolic parameters, whereas those with very low LVEF demonstrated a restrictive LV diastolic filling pattern. LV dysfunction was associated with segmental wall motion abnormalities in 72% of all patients, a finding similar in patients with low LVEF and those with very low LVEF. Apical akinesia was more common in patients with very low LVEF than in those with low LVEF.
| EF ≥50% (n = 32) | EF <36%–<50% (n = 16) | EF ≤35% (n = 16) | ||||
|---|---|---|---|---|---|---|
| Before AVR | After AVR | Before AVR | After AVR | Before AVR | After AVR | |
| Age (years) | 69 ± 11 | 76 ± 11 | 69 ± 10 | |||
| Men | 56% | 63% | 73% | |||
| Body surface area (m 2 ) | 1.8 ± 0.2 | 1.8 ± 0.2 | 1.9 ± 0.2 | |||
| Peak atrioventricular gradient (mm Hg) | 96 ± 28 | 39 ± 14 ⁎ | 94 ± 36 | 29 ± 9 ⁎ | 75 ± 28 ‡ | 31 ± 11 ⁎ |
| Aortic valve area (cm 2 ) | 0.8 ± 0.2 | 1.6 ± 0.3 ⁎ | 0.7 ± 0.2 | 1.7 ± 0.3 ⁎ | 0.8 ± 0.2 | 1.7 ± 0.4 ⁎ |
| Left ventricular diastolic diameter (cm) | 4.9 ± 0.5 | 4.7 ± 0.5 | 5.3 ± 0.6 † | 5.2 ± 0.5 | 5.6 ± 0.4 ‡ | 5.3 ± 0.6 |
| Left ventricular systolic diameter (cm) | 3.1 ± 0.5 | 3.0 ± 0.5 | 4.0 ± 0.5 † | 3.8 ± 0.6 | 4.8 ± 0.3 ‡ | 4.2 ± 0.8 |
| Septal thickness (cm) | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.4 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.2 | 1.3 ± 0.2 |
| Posterior wall thickness (cm) | 1.1 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.2 |
| Left ventricular mass (g) | 224 ± 56 | 206 ± 50 | 273 ± 91 † | 252 ± 63 | 252 ± 62 ‡ | 232 ± 56 |
| Left atrial systolic diameter (cm) | 4.1 ± 0.5 | 4.3 ± 0.4 | 4.5 ± 0.5 | 4.6 ± 0.6 | 4.7 ± 0.5 | 4.6 ± 0.6 |
| Left ventricular ejection fraction (%) | 67 ± 6 | 65 ± 4 | 43 ± 4 † | 56 ± 8 ⁎ | 28 ± 8 ‡ | 41 ± 9 ⁎ |
| Mitral inflow E/A ratio | 1.1 ± 0.7 | 1.1 ± 0.4 | 0.9 ± 0.6 | 1.4 ± 0.5 ⁎ | 2.3 ± 1.3 ‡ | 1.5 ± 0.4 ⁎ |
| E deceleration time (ms) | 254 ± 83 | 221 ± 61 | 234 ± 75 | 183 ± 44 ⁎ | 150 ± 48 ‡ | 190 ± 25 ⁎ |
| Wall motion abnormalities | 0 | 69% † | 75% ‡ | |||
| Apical wall motion abnormalities | 0 | 13% † | 58% ‡ | |||
| Coronary bypass graft during aortic valve replacement | 19% | 62% † | 75% ‡ | |||
⁎ p <0.05, before versus after aortic valve replacement.
† p <0.05, low versus normal left ventricular ejection fraction.
‡ p <0.05, very low versus low left ventricular ejection fraction.
For ease of understanding, changes in strain and strain rate are referred to in relation to their absolute values, i.e., greater strain denotes larger negative value (better function with increased shortening).
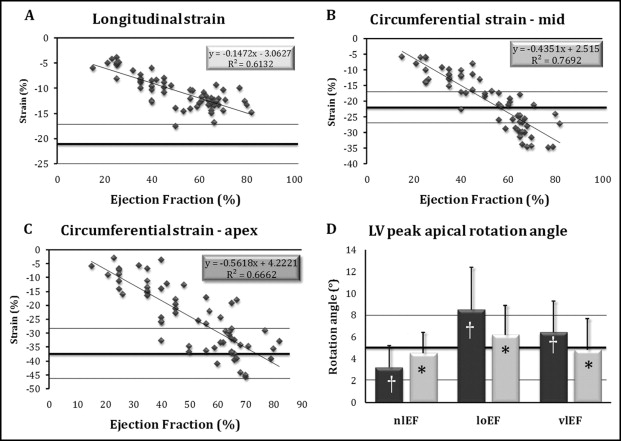
Average longitudinal strain was low in patients with normal LVEF and further decreased with lower LVEF ( Figure 1 , Table 2 ). Mid-LV circumferential strain was higher than normal in patients with normal LVEF, whereas it was low with low LVEF and continued to decrease with very low LVEF. Apical circumferential strain was in the normal range in patients with normal LVEF and gradually decreased in patients with low LVEF and very low LVEF. All strain components (longitudinal and mid-LV and apical circumferential) correlated significantly with LVEF (Spearman ρ = 0.74, 0.88, 0.74, respectively, p <0.001 for all correlations). Mid-circumferential strain vector calculated from its biplane components ( Figure 2 ) correlated with EF better than either component (Spearman ρ = 0.9, p <0.0001).
| Normal (n = 39) | EF ≥50% | EF <35%–<50% | EF ≤35% | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before AVR | After AVR | Percent Change | Before AVR | After AVR | Percent Change | Before AVR | After AVR | Percent Change | ||
| Strain (%) | ||||||||||
| Longitudinal | −21 ± 4 | −13 ± 2 | −16 ± 3 ⁎ | +29 ± 15 | −11 ± 3 † | −13 ± 2 ⁎ | +49 ± 30 | −7 ± 2 ‡ | −9 ± 2 ⁎ | +61 ± 40 |
| Circumferential—mid | −22 ± 4 | −27 ± 5 | −22 ± 5 ⁎ | −14 ± 23 | −15 ± 4 † | −18 ± 5 ⁎ | +34 ± 69 | −10 ± 3 ‡ | −14 ± 4 ⁎ | +50 ± 75 |
| Circumferential—apex | −35 ± 8 | −33 ± 7 | −31 ± 7 | −3 ± 3 | −22 ± 9 † | −27 ± 7 ⁎ | +45 ± 66 | −10 ± 4 ‡ | −19 ± 5 ⁎ | +134 ± 147 |
| Strain rate (%/s) | ||||||||||
| Longitudinal systolic | −1.1 ± 0.1 | −0.6 ± 0.1 | −0.8 ± 0.1 ⁎ | +35 ± 23 | −0.6 ± 0.1 | −0.8 ± 0.2 ⁎ | +37 ± 36 | −0.4 ± 0.1 ‡ | −0.6 ± 0.1 ⁎ | +54 ± 41 |
| Circumferential systolic (mid) | −1.2 ± 0.4 | −1.3 ± 0.5 | −1.1 ± 0.3 | −14 ± 27 | −0.8 ± 0.2 † | −1.1 ± 0.4 ⁎ | +53 ± 79 | −0.5 ± 0.2 ‡ | −0.8 ± 0.2 ⁎ | +58 ± 76 |
| Circumferential systolic (apex) | −2.1 ± 0.6 | −1.8 ± 0.5 | −1.8 ± 0.5 | +5 ± 35 | −1.3 ± 0.6 † | −1.8 ± 0.4 ⁎ | +57 ± 61 | −0.6 ± 0.3 ‡ | −1.2 ± 0.4 ⁎ | +157 ± 180 |
| Longitudinal early diastolic | 1.0 ± 0.3 | 0.5 ± 0.1 | 0.7 ± 0.2 ⁎ | +40 ± 30 | 0.6 ± 0.1 | 0.7 ± 0.1 | +25 ± 33 | 0.4 ± 0.1 ‡ | 0.5 ± 0.1 ⁎ | +46 ± 34 |
| Circumferential early diastolic (mid) | 1.5 ± 0.4 | 1.2 ± 0.5 | 1.0 ± 0.3 | −19 ± 25 | 0.6 ± 0.1 † | 0.8 ± 0.3 ⁎ | +36 ± 50 | 0.6 ± 0.2 ‡ | 0.7 ± 0.2 ⁎ | +41 ± 67 |
| Circumferential early diastolic (apex) | 1.8 ± 0.5 | 1.5 ± 0.5 | 1.6 ± 0.6 | +11 ± 43 | 1.3 ± 0.5 | 1.6 ± 0.5 ⁎ | +51 ± 99 | 0.6 ± 0.3 ‡ | 1.0 ± 0.4 ⁎ | +110 ± 156 |
| Peak twist angle (°) | 8 ± 4 | 3.8 ± 2.2 | 6.1 ± 2.6 ⁎ | +127 ± 155 | 8.8 ± 3.3 † | 7.8 ± 3.2 | −7 ± 42 | 5.7 ± 2.9 ‡ | 6.4 ± 3.7 | +13 ± 59 |
| Apical rotation (°) | 5 ± 3 | 3.2 ± 2.0 | 4.5 ± 1.9 ⁎ | +83 ± 110 | 8.5 ± 3.9 † | 6.6 ± 2.7 ⁎ | −16 ± 46 | 6.4 ± 2.9 ‡ | 4.8 ± 2.9 ⁎ | −36 ± 27 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


