Increased neovascularization in atherosclerotic plaques is associated with plaque vulnerability. The high resolution of optical coherence tomography (OCT) might provide a chance to directly visualize plaque neovascularization in vivo. The aim of the present study was to investigate the relation between microchannels in culprit plaques identified by OCT and plaque vulnerability in patients with coronary artery disease. A total of 63 consecutive patients with coronary artery disease who had undergone both OCT and intravascular ultrasound before any interventions to examine culprit lesion morphologies were enrolled. Microchannel was defined as a no-signal tubuloluminal structure on the cross-sectional optical coherence tomographic image. Microchannels were found in 24 (38%) of the 63 patients. The patients were divided into 2 groups according to the presence or absence of microchannels. The frequency of plaque rupture tended to be greater in the microchannel group (50% vs 28%, p = 0.11). The thickness of the fibrous cap (median 60 vs 100 μm, p = 0.001) was significantly less in the patients with microchannels, and significant differences were found in the frequency of thin-cap fibroatheroma (54% vs 21%, p = 0.012) and positive remodeling (67% vs 36%, p = 0.02) between the 2 groups. The high-sensitivity C-reactive protein levels in the microchannel group was significantly greater than those in the no-microchannel group (median 0.27 vs 0.13 mg/dl, p = 0.015). Moreover, increased microchannel counts were associated with greater high-sensitivity C-reactive protein levels (p = 0.01). In conclusion, a significant relation was found between the presence of microchannels in plaques identified by OCT and plaque vulnerability in patients with coronary artery disease.
Optical coherence tomography (OCT) is an intracoronary imaging modality with a high resolution of 10 to 20 μm that provides detailed microstructural information as well as the histologic examination. OCT has the potential to identify angioneogenesis. Therefore, the high resolution of OCT might offer an opportunity to study the spatial distribution of plaque neovascularization in vivo. The aim of the present study was to investigate whether microchannel structures in culprit plaques identified by OCT are related to plaque vulnerability in patients with coronary artery disease.
Methods
A total of 74 patients with symptomatic stable angina pectoris or unstable angina pectoris who underwent both OCT and intravascular ultrasound (IVUS) examinations before percutaneous coronary intervention were consecutively enrolled in the present study. Stable angina pectoris was defined as chest pain on exertion, positive nuclear stress test results, and no change in the frequency, duration, or intensity of symptoms within ≥4 weeks before the intervention. Unstable angina pectoris was defined as angina at rest, accelerated angina, or new-onset angina. The exclusion criteria were as follows: (1) a history of previous myocardial infarction; (2) left main trunk disease; (3) chronic total occlusion; (4) extremely tortuous or calcified vessels with expected difficulty in advancing the OCT catheter; (5) target vessel reference diameter of ≥4 mm expected limitation in OCT imaging; (6) congestive heart failure with left ventricular ejection fraction <40%; (7) renal insufficiency with baseline serum creatinine >1.5 mg/dl; (8) inflammatory disease (eg, infection, autoimmune disease); and (9) secondary angina caused by anemia.
Seven patients were excluded because the OCT examination was not performed according to the exclusion criteria (n = 4), the imaging quality precluded analysis (n = 2), or the equipment malfunctioned (n = 1). Another 4 patients were also excluded because the IVUS catheter failed to pass through the lesion before percutaneous coronary intervention. Finally, we analyzed the remaining 63 patients with successful OCT and IVUS imaging results. Of the 63 patients, 44 presented with unstable angina pectoris and 19 with stable angina pectoris. All demographic and clinical data were collected prospectively.
The ethics committee of Wakayama Medical University approved the study protocol. All patients provided written informed consent before participation in the study.
Coronary angiography was performed with a 5Fr Judkins-type catheter using the conventional femoral approach. All patients received an intravenous bolus injection of 2,000 IU of unfractionated heparin and intracoronary isosorbide dinitrate (2 mg) before angiography. The culprit lesion for each patient was identified from the findings of coronary angiography, electrocardiography, nuclear stress test, and transthoracic echocardiography.
After coronary angiography, to observe the culprit lesion before any interventions, OCT and IVUS imaging were performed using a 6Fr sheath and guiding catheter.
Oral aspirin (162 mg) and intravenous unfractionated heparin (8,000 IU) were administered before the percutaneous coronary intervention. A 0.016-in. OCT catheter (ImageWire, LightLab Imaging, Westford, Massachusetts) was advanced to the distal end of the culprit lesion through a 3Fr (distal) occlusion balloon catheter (Helios, Goodman, Nagoya, Japan). To remove the blood cells from the imaging field, the occlusion balloon was inflated to 0.5 atm proximal to the culprit lesion, and lactated Ringer’s solution was infused into the coronary artery from the distal tip of the occlusion balloon catheter at a rate of 0.5 ml/s, as reported previously.
For proximal lesions, we used a continuous-flushing (nonocclusive) technique of OCT image acquisition, a newly developed alternative to the balloon-occlusion technique. To flush the vessel, we infused a mixture of commercially available dextran 40 and lactated Ringer’s solution (low-molecular-weight dextran L Injection, Otsuka Pharmaceutical Factory, Tokushima, Japan) direct from the 6Fr guiding catheter at a rate of 2.5 to 4.5 ml/s using an injector pump (Mark V, Medrad, Warrendale, Pennsylvania). Regardless of the OCT technique used, in all cases, the entire length of the culprit lesion was imaged using an automatic pullback device moving at 1 mm/s. The OCT images were recorded digitally and analyzed using the M2CV (musical instrument to digital interface to control voltage converter) OCT console.
All OCT images were analyzed by 2 independent investigators who were unaware of the clinical presentations. Any discrepancies between the observers were resolved by consensus. The presence of plaque rupture, intracoronary thrombus, or thin-cap fibroatheroma (TCFA) was recorded. Plaque rupture was defined as the presence of fibrous cap discontinuity and cavity formation in the plaque. Intracoronary thrombus was identified as a mass protruding into the vessel lumen from the surface of the vessel wall. The OCT images were analyzed using previously validated criteria for plaque characterization, and the fibrous cap thickness was determined, as previously reported.
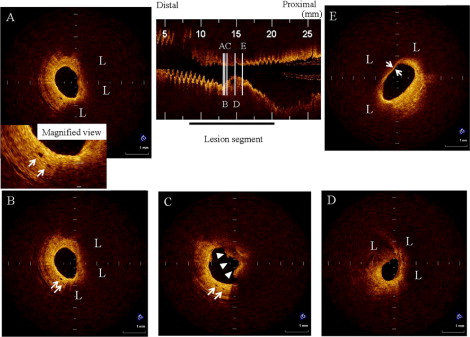
Lipid was semiquantified according to the number of involved quadrants on the cross-sectional OCT image. When lipid was present in ≥2 quadrants in any of the images within a plaque, it was considered a lipid-rich plaque. For each patient, the cross-sectional image with the greatest number of lipid quadrants was used for analysis. TCFA was defined as a plaque with lipid content in ≥2 quadrants and the thinnest part of the fibrous cap measuring <70 μm. A microchannel was defined as a no-signal tubuloluminal structure without a connection to the vessel lumen recognized on ≥3 consecutive cross-sectional OCT images ( Figure 1 ). The number of microchannels was also counted at the maximum site.
The IVUS (Eagle Eye, Volcano Therapeutics, San Diego, California) examination was performed with an automatic pullback device at a rate of 0.5 mm/s. All IVUS images were recorded digitally and analyzed by 2 independent observers who were unaware of the OCT data. When a discordance occurred between the observers, a consensus reading was obtained. The corresponding OCT and IVUS images were identified by the distances from 2 landmarks, such as side branches and/or calcifications. The IVUS analysis was done according to the criteria of the American College of Cardiology Clinical Expert Consensus Document on IVUS evaluations. The external elastic membrane, lumen, and plaque plus media cross-sectional area and longitudinal lesion length were measured. The plaque burden was calculated as the plaque plus media cross-sectional area divided by the lesion external elastic membrane cross-sectional area. The references were the single slice with the largest lumen and smallest plaque burden within 5 mm proximally. The remodeling index was calculated as the lesion external elastic membrane cross-sectional area divided by the proximal reference cross-sectional area. Positive remodeling was defined as a remodeling index >1.05.
Blood was collected on admission. The blood samples were centrifuged, and the serum was removed and stored at −80°C until the assays could be performed. High-sensitivity C-reactive protein (hs-CRP) was analyzed using a commercially available testing kit (N-Latex CRP II, Dade Behring Marburg GmbH, Marburg, Germany).
All statistical analyses were performed using StatView, version 5.0J (SAS Institute, Cary, North Carolina). Continuous variables are expressed as the mean ± SD or median (interquartile range). Categorical data are presented as numbers (%). Continuous variables were compared using an unpaired Student’s t test or the Mann-Whitney U test and categorical data using the chi-square test or Fisher’s exact test, as appropriate. When classified into 3 groups according to the number of microchannels, differences among the groups for hs-CRP levels were assessed using the Kruskal-Wallis test, because the hs-CRP level was not normally distributed. The frequency of positive remodeling or TCFA was compared among the groups using the chi-square test or Fisher’s exact test. P Values <0.05 were considered statistically significant.
Results
In patients with unstable angina pectoris, the mean interval between symptom onset and OCT imaging was 1.7 ± 0.8 days. Microchannels were observed in 20 (45%) of 44 patients with unstable angina pectoris and 4 (21%) of 19 patients with stable angina pectoris. When the lesion segment was divided into 3 equal intervals, microchannels were found at the proximal site of the lesion segment in 8 (33%) of 24 patients, the distal site in 14 (58%), and the minimum lumen area site in 2 (8%). The patients were divided into those with microchannels (n = 24) and those without microchannels (n = 39).
The patient characteristics at baseline are listed in Table 1 . Although no significant differences were found between the 2 groups in terms of age, gender, clinical presentation, coronary risk factors, distribution of the culprit coronary artery, lipid profiles, leukocyte count, or hemoglobin A1c, the hs-CRP levels in the microchannel group were significantly greater than those in the no-microchannel group. Moreover, greater hs-CRP levels were associated with increased microchannel counts (p = 0.01; Figure 2 ).
| Variable | Microchannels | p Value | |
|---|---|---|---|
| Yes (n = 24) | No (n = 39) | ||
| Age (years) | 64 ± 11 | 67 ± 11 | 0.30 |
| Men | 15 (63%) | 33 (85%) | 0.07 |
| Clinical presentation | 0.09 | ||
| Unstable angina pectoris | 20 (83%) | 24 (62%) | |
| Stable angina pectoris | 4 (17%) | 15 (38%) | |
| Coronary risk factors | |||
| Hypertension | 20 (83%) | 27 (69%) | 0.25 |
| Diabetes mellitus | 7 (29%) | 12 (31%) | 1.00 |
| Total cholesterol >220 mg/dl | 13 (54%) | 17 (44%) | 0.45 |
| Smoker | 10 (42%) | 16 (41%) | 1.00 |
| Culprit coronary artery | |||
| Left anterior descending | 13 (54%) | 23 (59%) | 0.80 |
| Left circumflex | 4 (17%) | 6 (15%) | 1.00 |
| Right | 7 (29%) | 10 (26%) | 0.78 |
| Leukocyte count (/μl) | 6,552 ± 1409 | 6,313 ± 2151 | 0.63 |
| Total cholesterol (mg/dl) | 200 ± 48 | 191 ± 40 | 0.43 |
| Low-density lipoprotein cholesterol (mg/dl) | 124 ± 46 | 118 ± 29 | 0.53 |
| High-density lipoprotein cholesterol (mg/dl) | 47 ± 13 | 46 ± 15 | 0.79 |
| Triglycerides (mg/dl) | 130 ± 84 | 123 ± 57 | 0.69 |
| Hemoglobin A1c (%) | 5.7 ± 0.9 | 5.8 ± 1.3 | 0.74 |
| High-sensitivity C-reactive protein (mg/dl) | 0.27 (0.18–0.52) | 0.13 (0.10–0.30) | 0.015 |

The IVUS findings are listed in Table 2 . The frequency of positive remodeling was significantly greater in patients with microchannels (67% vs 36%, p = 0.02). Furthermore, when categorized into 3 groups according to the number of microchannels, a significant difference was found in the frequency of positive remodeling (36%, 50%, and 79%, in the group with 0, 1, and ≥2 microchannels, respectively; p <0.007; Figure 3 ). The frequency of positive remodeling was significantly different between the group with ≥2 and 0 microchannels (p = 0.011) but not between the group with 0 and 1 microchannel (p = 0.480) or the group with ≥2 and 1 microchannels (p = 0.204).
| Variable | Microchannels | p Value | |
|---|---|---|---|
| Yes (n = 24) | No (n = 39) | ||
| Reference site | |||
| External elastic membrane cross-sectional area (mm 2 ) | 14.7 ± 3.2 | 14.0 ± 2.5 | 0.34 |
| Lumen cross-sectional area (mm 2 ) | 7.4 ± 2.6 | 6.7 ± 1.5 | 0.18 |
| Plaque plus media cross-sectional area (mm 2 ) | 7.3 ± 3.2 | 7.4 ± 2.9 | 0.90 |
| Lesion site | |||
| External elastic membrane cross-sectional area (mm 2 ) | 15.9 ± 3.9 | 14.4 ± 2.9 | 0.09 |
| Lumen cross-sectional area (mm 2 ) | 2.5 ± 0.6 | 2.9 ± 1.2 | 0.13 |
| Plaque plus media cross-sectional area (mm 2 ) | 13.4 ± 3.6 | 11.5 ± 3.3 | 0.04 |
| Lesion length (mm) | 21.8 ± 11.6 | 23.0 ± 9.7 | 0.66 |
| Plaque burden (%) | 83.6 ± 5.6 | 78.6 ± 10.8 | 0.037 |
| Positive remodeling | 16 (67%) | 14 (36%) | 0.02 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


