Elevated serum uric acid (SUA) level is known to be a prognostic factor in patients with acute coronary syndrome (ACS). However, the pathogenesis of the relation between SUA level and coronary plaque characteristics has not been fully evaluated. The aim of this study was to investigate the relation between SUA level and plaque composition of nonculprit lesions in patients with ACS. A total of 81 patients with ACS who underwent intravascular ultrasound (IVUS)–guided percutaneous coronary intervention were included. They were classified into 3 groups according to tertiles of SUA level. Using integrated backscatter (IB)–IVUS system, tissue components were classified into 4 categories: calcium deposits, dense fibrosis, fibrosis, and lipid. Tertiles of SUA level were as follows: low tertile <5.0 mg/dl; intermediate tertile 5.0 to 6.4 mg/dl; and high tertile >6.4 mg/dl. There was a trend toward greater vessel volume in the high tertile group than in the low and intermediate tertile groups (19.4 ± 3.7 vs 17.4 ± 4.4 vs 16.7 ± 4.1 mm 3 /mm, p = 0.05). There was no significant difference in lumen volume between the 3 groups. Plaque volume was significantly greater in the high than in the low tertile group (8.6 ± 2.4 vs 6.7 ± 2.2 mm 3 /mm, p = 0.01). IB-IVUS analysis demonstrated greater lipid (59.1 ± 9.1% vs 49.7 ± 10.9% vs 51.1 ± 9.3%, p = 0.001) and less fibrous components (36.8 ± 7.8% vs 44.3 ± 7.8% vs 43.2 ± 6.7%, p <0.001) in the high than in the low and intermediate tertile groups. Multivariate analysis showed high SUA as an independent predictor of increasing lipid volume. In conclusion, elevated SUA level is associated with greater lipid content of coronary plaque in patients with ACS than in patients with normal levels.
Elevated serum uric acid (SUA) level is associated with cardiovascular events in patients with coronary risk factors such as hypertension or diabetes. It is also a prognostic factor in patients with acute coronary syndrome (ACS), especially ST-segment elevation myocardial infarction. However, the pathogenesis of the relation between SUA level and coronary plaque characteristics has not been fully evaluated. Integrated backscatter-intravascular ultrasound (IB-IVUS) allows quantitative detection of coronary plaque components in vivo. Increased lipid-rich plaque is an independent predictor of cardiovascular events after percutaneous coronary intervention (PCI). It is also associated with no-reflow and slow-flow phenomenon during PCI. The aim of this study was to investigate the relation between SUA level and plaque composition of nonculprit lesions in patients with ACS.
Methods
From April 2012 to December 2014, a total of 313 patients with ACS underwent PCI at Chiba University Hospital. Patients were considered eligible for this study when SUA measurement on admission was available and IVUS-guided PCI was performed. The major criteria for exclusion were patients receiving antihyperuricemic agents before admission, hemodialysis, no stent implantation, emergent surgery or mechanical support (e.g., extracorporeal membrane oxygenation), cardiopulmonary arrest, inhospital adverse events, and a target lesion in stented segment. Patients who had no analyzable nonculprit plaque (plaque burden >20% and at least 5-mm length) were also excluded. Thus, 81 patients were included in the study ( Figure 1 ). They were classified into 3 groups according to tertiles of SUA level.
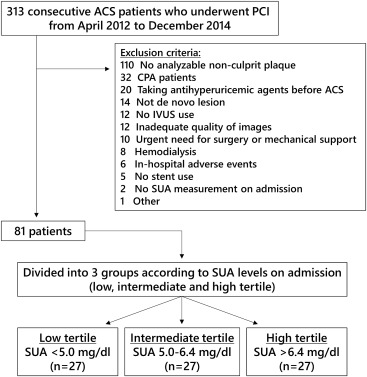
ACS was defined as unstable angina or acute myocardial infarction (MI) <48 hours from the onset. The diagnosis of acute MI was based on the third universal definition of MI. Unstable angina was diagnosed using Braunwald criteria plus documentation of significant coronary artery disease on coronary angiography. The ethics committee of Chiba University approved the study.
All grayscale IVUS and IB-IVUS were performed after intracoronary administration of isosorbide dinitrate 1 to 2 mg. They were acquired with a commercially available IVUS imaging system (VISIWAVE, Terumo, Tokyo, Japan) using a 43-MHz mechanically rotating IVUS catheter (View IT, Terumo) with a motorized transducer pullback speed of 0.5 mm/s. All IVUS measurements were performed by an experienced investigator, who was unaware of the patients’ clinical characteristics, according to the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement, and Reporting of Intravascular Ultrasound Studies. Offline analysis including IB-IVUS analysis was performed using a computerized system (VISIATLAS, Terumo, Tokyo, Japan). IVUS measurement was performed from the ostium of the culprit artery to the site 5-mm proximal to the proximal edge of the stent. For each 0.5 mm of axial length, lumen and external elastic membrane cross-sectional areas were manually measured. Volumetric IVUS data were presented as total volume per lesion length (mm 3 /mm) for correcting the differences of lesion length. Tissue components were classified into 4 categories: calcium deposits (red), dense fibrosis (yellow), fibrosis (green), and lipid (blue) according to signal level. The percentage of lipid, fibrosis, dense fibrosis, and calcium deposits plaque volume (each component volume/plaque volume × 100) were calculated.
Statistical analysis was performed with SAS statistical software package version 9.4 (SAS Institute, Cary, North Carolina). Data are expressed as the mean ± standard deviation or frequency (%). Continuous variables were compared using analysis of variance. Categorical variables were compared with the chi-square statistics or Fisher’s exact test. A value of p <0.05 was considered significant. Univariate analysis for variables in Table 1 was performed using linear regression analysis of rank-transformed outcomes. Including only variables with a p value of <0.2 on univariate analysis, multivariate analysis was performed using multiple linear regression analysis of rank-transformed outcomes.
| Variable | Tertile | p Value | ||
|---|---|---|---|---|
| Low (n = 27) | Intermediate (n = 27) | High (n = 27) | ||
| Men | 17 (63%) | 24 (89%) | 25 (93%) | 0.01 |
| Age (years) | 65.5 ± 9.2 | 66.0 ± 14.3 | 63.8 ± 12.7 | 0.79 |
| Body mass index (kg/m 2 ) | 23.3 ± 3.5 | 23.5 ± 3.2 | 25.2 ± 3.2 | 0.08 |
| Hypertension | 20 (74%) | 16 (59%) | 19 (70%) | 0.49 |
| Diabetes mellitus | 11 (41%) | 7 (26%) | 9 (33%) | 0.59 |
| Dyslipidemia | 17 (63%) | 19 (70%) | 20 (70%) | 0.68 |
| Current smoker | 10 (37%) | 11 (41%) | 11 (41%) | 0.95 |
| Prior myocardial infarction | 2 (7%) | 2 (7%) | 3 (11%) | 0.86 |
| eGFR (ml/min/1.73m 2 ) | 85.1 ± 20.9 | 72.0 ± 17.6 | 60.3 ± 18.5 | <0.001 |
| Serum uric acid (mg/dl) | 4.0 ± 1.0 | 5.5 ± 0.4 | 7.6 ± 1.1 | <0.001 |
| Culprit coronary artery | ||||
| Right | 7 (26%) | 7 (26%) | 10 (37%) | |
| Left anterior descending | 10 (37%) | 12 (44%) | 13 (48%) | |
| Left circumflex | 10 (37%) | 8 (30%) | 4 (15%) | 0.45 |
| Clinical presentation | ||||
| Unstable angina pectoris | 3 (11%) | 5 (19%) | 4 (15%) | |
| NSTEMI | 8 (30%) | 9 (33%) | 14 (52%) | |
| STEMI | 16 (59%) | 13 (48%) | 9 (33%) | 0.35 |
| Medication on admission | ||||
| Statin | 8 (30%) | 9 (33%) | 9 (33%) | 0.95 |
| ACE-I or ARB | 7 (26%) | 13 (48%) | 11 (41%) | 0.24 |
| Calcium channel blocker | 8 (30%) | 7 (26%) | 7 (26%) | 0.94 |
| Diuretic | 1 (4%) | 1 (4%) | 5 (19%) | 0.08 |
Results
Tertiles of SUA level were as follows: low tertile <5.0 mg/dl; intermediate tertile 5.0 to 6.4 mg/dl; and high tertile >6.4 mg/dl. Table 1 lists baseline characteristics. Table 2 provides IVUS measurements. IB-IVUS analysis demonstrated greater lipid and less fibrous components in the high tertile group than in the low and intermediate tertile groups ( Figure 2 ). Figure 3 shows the representative IVUS images in patients with low and high SUA levels. Multivariate analysis showed high SUA level as an independent predictor of greater lipid plaque volume ( Table 3 ).
| Variable | Tertile | p Value | ||
|---|---|---|---|---|
| Low (n = 27) | Intermediate (n = 27) | High (n = 27) | ||
| Lesion length (mm) | 15.6 ± 10.1 | 20.0 ± 12.6 | 14.2 ± 10.1 | 0.14 |
| Minimum lumen area (mm 2 ) | 8.2 ± 2.9 | 6.1 ± 2.6 | 7.6 ± 3.8 | 0.05 |
| Vessel area (mm 2 ) | 16.4 ± 4.3 | 14.4 ± 5.0 | 17.5 ± 4.8 | 0.06 |
| Plaque area (mm 2 ) | 8.3 ± 3.6 | 8.3 ± 3.9 | 9.8 ± 3.4 | 0.22 |
| Lumen volume (mm 3 /mm) | 10.7 ± 3.2 | 9.9 ± 3.1 | 10.6 ± 3.2 | 0.60 |
| Vessel volume (mm 3 /mm) | 17.4 ± 4.4 | 16.7 ± 4.1 | 19.4 ± 3.7 | 0.05 |
| Plaque volume (mm 3 /mm) | 6.7 ± 2.2 | 7.4 ± 2.7 | 8.6 ± 2.4 | 0.01 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


