Major vascular complications (VC) remain frequent after transcatheter aortic valve implantation (TAVI) and may be associated with unfavorable clinical outcomes. The objective of this study was to evaluate the rate of VC after transfemoral TAVI performed using an exclusive open surgical access strategy. From 2010 to 2014, we included in a monocentric registry all consecutive patients who underwent transfemoral TAVI. The procedures were performed with 16Fr to 20Fr sheath systems. VC were evaluated within 30 days and classified as major or minor according to the Valve Academic Research Consortium 2 definition. The study included 396 patients, 218 were women (55%), median age was 85 years (81 to 88), and the median logistic Euroscore was 15.2% (11 to 23). The balloon-expandable SAPIEN XT and the self-expandable Medtronic Core Valve prosthesis were used in 288 (72.7%) and 108 patients (27.3%), respectively. The total length of the procedure was 68 ± 15 minutes including 13 ± 5 minutes for the open surgical access. Major and minor VC were observed in 9 (2.3%) and 16 patients (4%), respectively, whereas life-threatening and major bleeding concerned 18 patients (4.6%). The median duration of hospitalization was 5 days (interquartile range 2 to 7), significantly higher in patients with VC (7 days [5 to 15], p <0.001). Mortality at 1-month and 1-year follow-up (n = 26, 6.6%; and n = 67, 17.2%, respectively) was not related to major or minor VC (p = 0.6). In multivariable analysis, only diabetes (odds ratio 2.5, 95% confidence interval 1.1 to 6.1, p = 0.034) and chronic kidney failure (odds ratio 3.0, 95% confidence interval 1.0 to 9.0, p = 0.046) were predictive of VC, whereas body mass index, gender, Euroscore, and lower limb arteriopathy were not. In conclusion, minimal rate of VC and bleeding can be obtained after transfemoral TAVI performed using an exclusive surgical strategy, with a particular advantage observed in high-risk bleeding patients.
Transcatheter aortic valve implantation (TAVI) is currently a valid option to surgery in patients with severe symptomatic aortic stenosis who are contraindicated or at high risk to surgical replacement. Although alternative access sites have been developed since the introduction of the technique by Cribier et al in 2001, the transfemoral route remains the most widely used for this procedure. Despite an improvement in operator experience, valve technology, and better screening, major vascular complications (VC) remain frequent and may be associated with unfavorable clinical outcomes with significantly higher rate of major bleeding and correlated with 1-year all-cause and cardiac mortality. According to the Valve Academic Research Consortium 2 (VARC-2) criteria, major VC are still reported with an incidence of 3% to 15% of the procedures, the low rates observed usually in registries. Although percutaneous (PC) closure has been routinely adopted by many centers and performed with a high rate of success after TAVI, the open surgical (OS) access is still widely used, and the superiority of one approach (e.g., surgical cut down) compared with the other (e.g., closure device) still remains a matter of debate. The OS approach may have been viewed as more predictable, offering more direct control during adverse events. Conversely, the PC access is considered as less invasive especially using small diameter sheaths and associated in some studies with significantly lower postprocedural length of stay than the surgical route. The objective of this study was to evaluate incidence of VC in a large population of patients when TAVI is performed with an exclusive femoral OS access and to identify predictive factors of VC using this strategy.
Methods
This monocentric observational study was conducted in the cardiology department of the Montpellier University Hospital, France, from January 2010 to February 2014. It included 396 consecutive patients who had a transfemoral TAVI procedure using the balloon-expandable Edwards SAPIEN XT bovine pericardial device (Edwards Life Sciences, Irvine, California) or the self-expandable Core Valve porcine pericardial device (Medtronic, Inc., Minneapolis, Minnesota). All patients had severe symptomatic aortic stenosis secondary to degenerative disease confirmed by transthoracic echocardiography (mean gradient >40 mm Hg and/or valve area <1 cm 2 ) and were not candidate for aortic valve replacement after internal discussions on the therapeutic options within the multidisciplinary heart team. Vascular access was evaluated before the procedure in all patients with multislice computerized tomography angiography of the entire aorta using vascular windows settings (Advantage windows, version 4.6; General Electric Workstation, Milwaukee, Wisconsin). As recommended in the guidelines, in case of circumferential calcification and femoral arterial diameter ≤6.5 mm, patients were excluded from the femoral approach. All procedures were done under general anesthesia. For the SAPIEN XT valve, the sets included the Novaflex delivery system and appropriate introducer sheaths (16Fr to 20Fr). The Medtronic Core Valve (sizes 26 or 29 mm) was delivered through a 30-cm-long 18Fr sheath (Check-Flo Introducer) from Cook Medical (Bloomington, Indiana) with an outer diameter of 7.3 mm (16Fr). All patients received 0.5 mg/kg of heparin at the time of introducing the femoral sheath to achieve an activated clotting time of >250 seconds. A balloon aortic valvuloplasty was systematically performed (balloon size: 20, 23, or 25 mm) before implantation of the prosthesis. We used 2 surgical strategies: before 2012, a conventional surgical approach with femoral artery controlled using vessel loop and arterial repair achieved using arterial cross-clamping and interrupted suture, and after 2012, an equivalent of “surgical preclose technique” to avoid arterial cross-clamping and avoid purse string effect. For both techniques, the femoral artery was exposed through a transversal groin incision. The anterior wall of the artery was cleaned from surrounding tissues up to a few centimeters. If uneven calcified plaques were found by palpation, a longer part was freed. Then, the most suitable site was chosen. At the end of the procedure, both ends of the sutures were gently pulled to obtain coaptation of the arterial wall and tied. When minimal damage was present, primary transverse closure of the artery was undertaken with polypropylene suture. In situations where arterial damage was more extensive, a pericardial patch or an appropriately sized Dacron graft was used. All patients underwent a controlateral venous and arterial femoral approach using 6Fr sheath to perform angiography, hemodynamic monitoring, and ventricular pacing. We used 6Fr closure devices (angioseal; St. Jude Medical, St. Paul, Minnesota) for the controlateral femoral artery. All procedures were performed in the same site by 6 medical teams including 4 different surgeons and 8 interventional cardiologists. An association of clopidogrel 75 mg and aspirin 75 mg without loading dose was introduced in all patients after the procedure except in those with indication of vitamin K antagonists or direct oral anticoagulant therapy who had only aspirin 75 mg. The vitamin K antagonists were always stopped before the procedure to obtain an INR <2 and reintroduced 1 or 2 days later. The direct anticoagulation therapy was stopped at least 2 days before TAVI. Primary end point concerned incidence and risk factors of VC defined by the modified VARC-2 classification and bleeding complications according to the Bleeding Academic Research Consortium definition. Secondary end points included total mortality and major events at 1-month and 1-year follow-up (VARC-2 criteria). Patients’ characteristics were presented using median and interquartile range (IQR 25–75 ) for continuous variables and frequencies and proportions for categorical variables. Groups (VC vs no VC) were compared using the Wilcoxon-Mann-Whitney test for continuous variables and the chi-square or Fisher’s test for categorical ones. To determine the relative importance of the covariates on the occurrence of VC, a multivariable analysis using logistic regression was performed. A backward selection of the variables was used, with an alpha-to-exit set at 0.10. Odds ratio (OR) and their 95% confidence intervals were calculated. The goodness-of-fit of the models was assessed using the Hosmer and Lemeshow chi-square test. Statistical analysis was performed using SAS, v.9, statistical software (SAS Institute, Cary, North Carolina).
Results
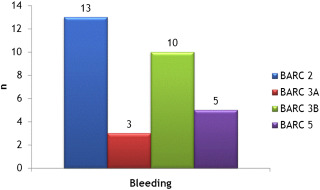
The study enrolled 396 patients, including 290 (73.2%) with a SAPIEN XT valve and 106 (26.8%) with a Medtronic Core Valve prosthesis. During the same period, 38 (7.6%) and 60 patients (12.1%) had TAVI through subclavian or transapical access, respectively. Patients’ characteristics are presented in Table 1 . The procedure was performed by the right femoral artery for 154 patients (38.9%) and through the left side for 242 of them (61.1%). The median total length of the procedure was 68 ± 19 minutes including 13 ± 5 minutes for the surgical access. At 1-month follow-up (primary end point), VC occurred in 25 patients (6.3%) including 9 (2.3%) with major and 16 (4%) with minor VC. Hematoma and femoral artery dissection were the 2 main complications observed ( Table 2 ). A surgical or a PC management of a VC was requisite for 9 patients (2.3%). Transfusions were required in 54 patients (13.7%), with only 1 unit of red blood cells (RBCs) needed for the vast majority of them (n = 44; 81.5%). Life-threatening and major bleeding concerned 18 patients (4.6%; Table 3 , Figure 1 ). RBC transfusions were inversely correlated to baseline hemoglobin level (11.4 ± 1.6 g/dl in patients with transfusion vs 12.5 ± 1.6 g/dl in those without transfusion, p <0.001). Predictive factors of VC evaluated in univariate analysis are presented in Table 4 . In multivariable analysis, only diabetes (OR 2.5; 95% confidence interval 1.1 to 6.1, p = 0.034) and chronic kidney failure (OR 3.0; 95% confidence interval 1.0 to 9.0, p = 0.047) were predictive of VC. Non-VC occurring at the access site, considered as specifically related to the use of the surgical approach, concerned 7 patients (1.8%) requiring surgery in 2 of them ( Table 5 ). Other complications occurring at 1-month follow-up (secondary end point) included mainly conduction disorders (n = 78, 19.7%) and required permanent pacing for 47 of them (11.9%) and acute kidney failure (n = 28, 7%). Ischemic stroke occurred in 4 patients (1%), for 3 of them during the inhospital stay. Acute heart failure concerned 10 patients (2.5%). Mortality at 1 month was 6.6% (n = 26) and was related to ventricular arrhythmias associated with left ventricular dysfunction (n = 3, 0.75%), annulus rupture (n = 4, 1%), mesenteric ischemia (n = 1, 0.25%), heart failure (n = 2, 0.5%), septic shock (n = 2, 0.5%), severe aortic regurgitation (n = 2, 0.5%), thoracic dissection (n = 1, 0.25%), or of unclear cause (n = 1, 0.25%). Mortality rate at 1-year follow-up was 17.3% (n = 67), related to cardiovascular cause in 12.6% of patients (n = 49), whereas 2% of the total population (n = 8) was lost at follow-up after index hospitalization. The occurrence of VC was not associated in our study with mortality at 1-month or 1-year follow-up. The need of RBC transfusions was not correlated to 1-month mortality (p = 0.7) but to mortality at 1-year follow-up (p = 0.02). Major VC in the controlateral access site were observed in 5 patients (1.2%). The median duration of hospitalization was 5 days (interquartile range 2 to 7), significantly higher in patients with VC (7 days [5 to 15], p <0.001).
| Female | 218 (55.1%) |
| Age, median (years) | 85 (81-88) |
| Body mass index, median (kg/m 2 ) | 25.2 (22.8-28.4) |
| Euroscore, median | 15.2 (11.0-23.0) |
| NYHA class | |
| I, II | 117 (30.4%) |
| III, IV | 268 (69.6%) |
| Hypertension | 247 (62.9%) |
| Diabetes mellitus | 103 (26.2%) |
| Smoker | 6 (1.5%) |
| Coronary artery disease | 202 (51.4%) |
| Peripheral arterial disease | 36 (9.2%) |
| Atrial fibrillation | 150 (38.2%) |
| Left ventricular ejection fraction | |
| ≤30% | 30 (7.7%) |
| 30-45% | 82 (21.0%) |
| >45% | 278 (71.3%) |
| Chronic kidney failure | 31 (7.9%) |
| Creatinine (μmol/L) median | 104 (81-129) |
| Hemoglobin (g/dL) median | 12.2 (11.3-13.4) |
| Albumin median (mg/l) | 40 (37-43) |
| Low molecular weight heparin | 44 (11.3%) |
| Vitamin K antagonist | 42 (10.8%) |
| Antiplatelet therapy | 293 (75.5%) |
| Double | 143 (36.7%) |
| Simple | 150 (38.7%) |
| Direct oral antithrombotic | 7 (1.8%) |
| Surgery period | |
| <2012 | 159 (40.2%) |
| ≥2012 | 237 (59.8%) |
| Vascular Complications | Major | Minor |
|---|---|---|
| False aneurysm | 1 (11.1%) | 0 |
| Hematoma | 2 (22.2%) | 13 (81.25%) |
| Fistula | 0 | 1 (6.25%) |
| Dissection | 5 (55.6%) | 1 (6.25%) |
| Femoral artery thrombosis | 0 | 1 (6.25%) |
| Thoracic aortic dissection | 1 (11.1%) | 0 |
| Total | 9 (2.3%) | 16 (4%) |
| 25 (6.3%) | ||
| Life threatening bleeding | Major bleeding | Minor bleeding | |
|---|---|---|---|
| Pericardial tamponnade | 13 (3.3%) | ||
| Related to annulus rupture | 6 (1.5%) | ||
| Hemorrhagic shock | 1 (0.25%) | ||
| Intracranial | 1 (0.25%) | ||
| During Aortic valve replacement surgery | 1 (0.25%) | ||
| Retroperitoneal hematoma | 1 (0.25%) | ||
| Unknown | 1 (0.25%) | ||
| Hematuria | 1 (0.25%) | ||
| Rectal hemorrage | 1 (0.25%) | ||
| Peri-procedural | 11 (2.8%) | ||
| Total | 15 (3.8%) | 3 (0.8%) | 13 (3.3%) |
| 31 (7.8%) | |||
| No vascular complications n = 371 | Vascular complications n = 25 | p | |
|---|---|---|---|
| Women | 205 (55.3%) | 13 (52.0%) | 0.75 |
| Age, median (years) | 85.0 (81.0-88.0) | 85.0 (83.0-88.0) | 0.27 |
| Body mass index, median | 25.1 (22.7-28.4) | 25.5 (23.1-29.7) | 0.64 |
| Euroscore, median | 15.5 (11.0-23.0) | 13.6 (11.89-22.3) | 0.71 |
| NYHA class | 1.00 | ||
| I, II | 110 (30.4%) | 7 (30.4%) | |
| III, IV | 252 (69.6%) | 16 (69.6%) | |
| Hypertension | 231 (62.6%) | 16 (66.7%) | 0.69 |
| Diabetes mellitus | 92 (24.9%) | 11 (47.8%) | 0.02 |
| Smoker | 6 (1.6%) | 0 | 0.53 |
| Coronary artery disease | 191 (51.8%) | 11 (45.8%) | 0.57 |
| Peripheral arterial disease | 35 (9.5%) | 1 (4.2%) | 0.71 |
| Atrial fibrillation | 139 (37. 74%) | 11 (45.8%) | 0.43 |
| Left ventricular ejection fraction | 0.63 | ||
| ≤ 30% | 29 (7.9%) | 1 (4.2%) | |
| 30-45% | 75 (20.5%) | 7 (29.2%) | |
| 45% | 262 (71.6%) | 16 (66.7%) | |
| Chronic kidney failure | 26 (7.0%) | 5 (20.8%) | 0.03 |
| Creatinine (μmol/L), median | 104.0 (81.0-127.0) | 110.0 (73.5-156.0) | 0.53 |
| Hemoglobin (g/dL), median (IQR 25-75 ) | 12.3(11.4-13.4) | 11.6 (10.3-12.8) | 0.10 |
| Low molecular weight heparin | 41 (11.3%) | 3 (12.5%) | 0.74 |
| Vitamin K antagonist | 39 (10.7%) | 3 (12.5%) | 0.73 |
| Antiplatelet therapy | 275 (75.6%) | 18 (75.0%) | 0.95 |
| DOA | 7 (1.9%) | 0 | 0.50 |
| Prosthesis | 0.43 | ||
| Edwards XT | 270 (72.8%) | 20 (80.0%) | |
| Corevalve | 101 (27.2%) | 5 (20.0%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


