Until now, few studies have examined QT intervals in subjects who consume alcohol. We performed this study to evaluate the associations between alcohol consumption and the QTc interval based on a general population. A total of 11,269 adults were examined using a multistage cluster sampling method to select a representative sample of subjects aged ≥35 years. Participants were asked to provide information about their alcohol consumption, and all participants received electrocardiograms and echocardiograms. A prolonged QTc interval was defined according to the national guidelines, which specify thresholds of ≥460 ms in women and ≥450 ms in men. Patients were divided into 3 categories, based on the amount of alcohol they consumed: heavy drinkers (>15 g/day for women and >30 g/day for men), moderate drinkers (≤15 g/day for women and ≤30 g/day for men), and nondrinkers (0 g/day). The results showed that the heavy drinkers had longer QTc intervals than did the nondrinkers. Multivariate logistic regression analyses revealed that men who were heavy drinkers had approximately 1.4-fold higher odds of having a prolonged QTc interval (odds ratio 1.431, 95% confidence interval [CI] 1.033 to 1.982, p = 0.031) than nondrinkers; in women, heavy drinkers had ∼2.3-fold higher odds of having a prolonged QTc interval (odds ratio 2.344, 95% CI 1.202 to 4.571, p = 0.012) than nondrinkers. Neither men nor women who were moderate drinkers exhibited a significant increase in risk for prolonged QTc interval. In conclusion, heavy alcohol consumption was found to be a risk factor for a prolonged QTc interval.
The QT interval has gained clinical importance because of its association with serious ventricular arrhythmia and sudden cardiac death. Prolonged QT intervals often occur because of either congenital or acquired abnormalities. Multiple factors have been implicated in both congenital and acquired QT prolongation, including older age, female gender, left ventricular (LV) hypertrophy, ischemia, slowed heart rate, and electrolyte abnormalities. Recently, laboratory studies have found that ethanol may inhibit the Kv1.5 channel currents and ventricular repolarization. Because the QT interval is the manifestation of ventricular depolarization and repolarization mediated through channels, these studies indicated the possibility that alcohol may be related to electrolyte abnormalities, which may cause QT interval prolongation. However, few studies have determined the relation between QT interval prolongation and alcohol consumption. Thus, we performed the present study in a general population with the hypothesis that alcohol consumption was associated with the QT interval.
Methods
From January 2012 to August 2013, a representative sample of subjects aged ≥35 years was selected to examine the prevalence, incidence, and history of cardiovascular risk factors in rural areas of Liaoning Province, China. The study adopted a multistage, stratified, random cluster sampling scheme. The detailed methods used in this study have been previously published. Because the population of subjects <35 years in China are more likely to migrate to other areas, we included participants ≥35 years to ensure sufficient response rates for a study of the general population. In all, 11,956 participants agreed to participate in the present study, and the response rate was 85.3%. The study was approved by the Ethics Committee of China Medical University (Shenyang, China). All procedures were performed in accordance with ethical standards. Written consent was obtained from all participants after they had been informed of the objectives, benefits, and medical factors. All participants signed a confidentiality agreement for protection of their personal information. If the participants were illiterate, we obtained the written, informed consent from their proxies. This study used baseline data, and only participants who had complete data regarding the analyzed variables were included, yielding a final sample size of 11,269 subjects (5,101 men and 6,168 women).
The data collection and measurement methods used in this study have been previously described. There was a central steering committee with a subcommittee for quality control. Data on demographic characteristics, lifestyle risk factors, dietary habits, family income, history of heart disease, and medication used in the past 2 weeks were obtained through an interview with a standardized questionnaire.
Fasting blood samples were collected from all participants in the morning after at least 12 hours of fasting. Serum potassium and magnesium, and other routine blood biochemical indexes, were analyzed enzymatically using an autoanalyzer (Olympus AU640 Auto-Analyzer; Olympus Corp., Kobe, Japan). All laboratory equipments were calibrated, and blinded, duplicate samples were used.
Standard 12-lead electrocardiograms (ECGs) were performed with a MAC 5500 (GE Health Care, Little Chalfont, Buckinghamshire, UK), as previously described, and the ECGs were analyzed automatically with a MUSE Cardiology Information System, version 7.0.0 (GE Health Care). ECG parameters, including the QT interval, were measured automatically. The QT interval is measured from the earliest detection of depolarization in any lead to the latest detection of repolarization in any lead. The error of QT interval measurements determined by an automatic algorithm was less than ±20 ms, the accuracy was 99.98%, and the sensitivity was 99.62%. Because the QT interval is influenced by heart rate, QT correction is required. In this study, the QTc interval was corrected for heart rate using Fridericia’s formula (QTc = QT/RR 1/3 ), and a prolonged QTc interval was defined according to the national guidelines, which specify thresholds of ≥460 ms in women and ≥450 ms in men.
The transthoracic ECG included M-mode, 2-dimensional, and color Doppler information. The details of the measurements used in this study have been previously published. LV internal diastolic dimension, interventricular septal thickness (IVST), and posterior wall thickness (PWT) were measured at the end of diastole and the end of systole according to the American Society of Echocardiography recommendations. LV mass (LVM) was calculated according to the American Society of Echocardiography simplified cubed equation : LVM (g) = 0.8 × {1.04 × [(LVIDd + PWTd + IVSTd) 3 − (LVIDd) 3 ]} + 0.6. LV mass was indexed for body surface area.
Alcohol consumption was assessed during each interview using the questionnaire. Participants were asked to provide information regarding whether they regularly consumed alcohol, their average amount of consumption per day, and the number of days per month that they consumed alcohol. The alcohol content of different types of beverages in China is known ; therefore, the amount of pure alcohol consumed was calculated based on the reported frequency and amount of drinking. In China, the ethanol weight content differs among beverages as follows: 5% in beer, 12.5% in red wine, and 45% in hard liquor. One drink was defined as an average of 15 g of ethanol. We used cutoff values based on the definition of daily alcohol consumption from the National Institute on Alcohol Abuse and Alcoholism to classify the levels of consumption: nondrinkers (abstainers, no alcohol consumption history), moderate drinkers (≤1 drink/day for women and ≤2 drinks/day for men), and heavy drinkers (>1 drink/day for women and >2 drinks/day for men).
Descriptive statistics were calculated for all the variables, including continuous variables (expressed as mean values and SDs) and categorical variables (expressed as numbers and proportions). Differences among categories were evaluated using the t test, analysis of variance, nonparametric tests, or the chi-square test, as appropriate. Comparisons between groups were performed using the Scheffe method. Multivariate logistic regression analyses were used to identify independent associations between alcohol consumption groups and prolonged QTc with calculated odds ratios (ORs) and corresponding 95% confidence intervals (CIs). All the statistical analyses were performed using SPSS, version 20.0, and p values <0.05 were considered to be statistically significant.
Results
The baseline characteristics of the included participants are listed in Table 1 . In total, 11,269 participants aged ≥35 years were included (5,101 men and 6,168 women). According to the definition of a prolonged QTc interval (thresholds of ≥460 ms in women and ≥450 ms in men), 4.2% of men and 4.5% of women had prolonged QTc intervals.
| Male (n=5101) | Female (n=6168) | P | |
|---|---|---|---|
| Age (years) | 54.4±10.83 | 53.4±0.35 | <0.001 |
| Race (Han) | 4833 (94.7%) | 5851 (94.9%) | 0.785 |
| Current smoker | 2910 (57.0%) | 1020 (16.5%) | <0.001 |
| Sleep duration (hours/day) | 7.4±1.63 | 7.1±1.74 | <0.001 |
| Activity level | <0.001 | ||
| Low | 1119 (21.9%) | 2214 (35.9%) | |
| Moderate | 3701 (72.6%) | 3601 (58.4%) | |
| High | 281 (5.50%) | 353 (5.70%) | |
| Body mass index (kg/m 2 ) | 24.7±3.55 | 24.9±3.77 | 0.041 |
| Heart rate (beats/min) | 69.9±12.37 | 73.3±12.44 | 0.85 |
| Prior stroke | 465 (9.30%) | 504 (8.30%) | 0.075 |
| Prior heart disease ∗ | 535 (10.7%) | 1144 (18.9%) | <0.001 |
| Medication used † | 2409 (47.6%) | 3568 (58.3%) | <0.001 |
| Total alcohol consumption (g/d) | 32.6±49.6 | 1.0±8.3 | <0.001 |
| Alcohol consumption from beer (g/d) | 3.9±12.8 | 0.1±2.0 | <0.001 |
| Alcohol consumption from liquor (g/d) | 28.6±46.9 | 0.9±7.7 | <0.001 |
| Alcohol consumption from wine (g/d) | 0.0±1.8 | 0.0±0.0 | 0.062 |
| Alcohol consumption | <0.001 | ||
| None | 2579 (50.6%) | 5966 (96.7%) | |
| Moderate | 766 (15.0%) | 78 (1.30%) | |
| Heavy | 1756 (34.4%) | 124 (2.00%) | |
| Corrected QT (Fridericia, ms) | 412.9±21.2 | 423.3±22.0 | <0.001 |
∗ Including coronary heart disease, arrhythmia and heart failure.
† Indicating any self-reported medication used in the past two weeks.
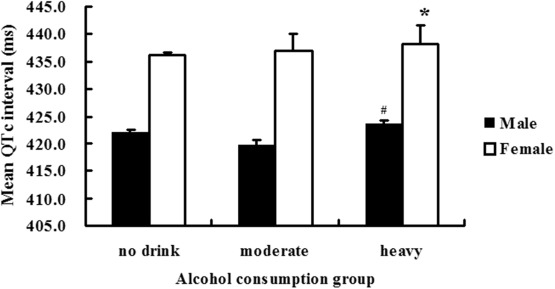
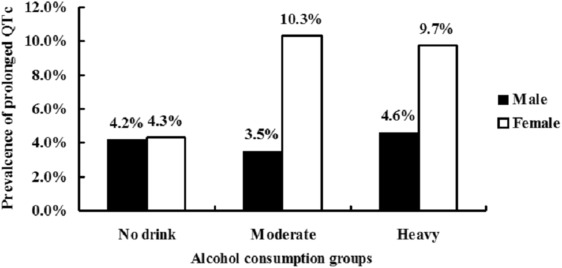
In men, the mean QTc intervals of heavy drinkers were longer than those of moderate and nondrinkers (412.46 ± 1.54 ms for nondrinkers, 411.43 ± 20.21 ms for moderate drinkers, and 414.22 ± 21.04 ms for heavy drinkers, p = 0.003). In women, the mean QTc intervals were also longer of heavy drinkers than those of moderate and nondrinkers (423.16 ± 21.70 ms for nondrinkers, 427.18 ± 24.93 ms for moderate drinkers, and 427.36 ± 32.84 ms for heavy drinkers, p = 0.032; Figure 1 ). As shown in Figure 2 , in women, heavy and moderate drinkers had a higher prevalence of prolonged QTc than nondrinkers (4.3% for nondrinkers, 10.3% for moderate drinkers, and 9.7% for heavy drinkers, p = 0.001). In men, the prevalence among the 3 alcohol consumption groups was 4.2% for nondrinkers, 3.5% for moderate drinkers, and 4.6% for heavy drinkers. There were no significant differences in the prevalence of QTc prolongation between the nondrinker and moderate drinker groups.
The characteristics of the study population according to QTc interval and gender are listed in Table 2 . Table 3 presents the results of the multivariate logistic regression analyses of the risk of prolonged QTc according to the different levels of alcohol consumption. After adjusting for the factors mentioned earlier, in men, heavy drinkers had approximately 1.4-fold higher odds of having a prolonged QTc interval (OR 1.431, 95% CI 1.33 to 1.982, p = 0.031) compared with nondrinkers. In women, heavy drinkers had ∼2.3-fold higher odds of having a prolonged QTc interval (OR 2.344, 95% CI 1.202 to 4.571, p = 0.012) compared with nondrinkers. Neither men nor women who were moderate drinkers exhibited a significantly increased risk of having a prolonged QTc interval.
| Variable | Male | Female | ||||
|---|---|---|---|---|---|---|
| Prolonged QTc | P | Prolonged QTc | P | |||
| No | Yes | No | Yes | |||
| Age (years) | 54.0±10.7 | 62.3±11.0 | <0.001 | 53.2±10.3 | 57.5±10.7 | <0.001 |
| Race (Han) | 4629 (94.8%) | 204 (94.4%) | 0.464 | 5586 (94.8%) | 265 (95.3%) | 0.785 |
| Current smoker | 2807 (57.5%) | 103 (47.7%) | 0.003 | 963 (16.3%) | 57 (20.5%) | 0.044 |
| Activity level | 0.002 | 0.851 | ||||
| Low | 1051 (21.5%) | 68 (31.5%) | 2116 (35.9%) | 98 (35.3%) | ||
| Moderate | 3563 (72.9%) | 138 (63.9%) | 3439 (58.4%) | 162 (58.3%) | ||
| High | 271 (5.5%) | 10 (4.6%) | 335 (5.7%) | 18 (6.5%) | ||
| Sleep duration (hours/day) | 7.4±1.6 | 7.4±1.9 | 0.926 | 7.1±1.7 | 7.1±1.8 | 0.546 |
| Left ventricular mass index (g/m 2 ) | 91.6±61.6 | 102.8±29.6 | 0.009 | 82.2±56.4 | 92.7±72.6 | 0.004 |
| Posterior wall thickness (mm) | 0.9±0.3 | 0.9±0.1 | 0.096 | 0.8±0.3 | 0.9±0.6 | 0.020 |
| Left ventricular internal diastolic dimension (mm) | 4.9±0.4 | 5.0±0.6 | 0.001 | 4.5±0.4 | 4.7±0.5 | <0.001 |
| Interventricular septal thickness (mm) | 0.9±0.3 | 1.0±0.2 | 0.001 | 0.9±0.3 | 0.9±0.1 | 0.032 |
| Heart rate (beats/min) | 70.0±12.4 | 68.0±12.2 | 0.019 | 73.5±12.5 | 68.7±9.5 | <0.001 |
| Alcohol consumption (g/day) | 32.4±49.6 | 35.1±50.7 | 0.429 | 1.0±8.2 | 2.2±10.4 | 0.060 |
| Serum magnesium (mmol/L) | 0.8±0.1 | 0.9±0.1 | 0.020 | 0.8±0.1 | 0.9±0.1 | 0.118 |
| Serum sodium (mmol/L) | 141.2±2.3 | 140.9±2.2 | 0.200 | 141.2±2.1 | 141.7±2.4 | 0.021 |
| Serum potassium (mmol/L) | 4.2±0.3 | 4.1±0.4 | 0.002 | 4.2±0.3 | 4.1±0.4 | <0.001 |
| Serum calcium (mmol/L) | 2.3±0.1 | 2.3±0.1 | 0.635 | 2.3±0.1 | 2.4±0.1 | 0.001 |
| Body mass index (kg/m 2 ) | 24.7±3.6 | 24.6±3.2 | 0.750 | 24.8±3.8 | 25.4±3.9 | 0.009 |
| Left ventricular ejection fraction | 0.6±0.1 | 0.6±0.1 | 0.759 | 0.6±0.1 | 0.6±0.1 | 0.502 |
| Prior stroke | 420 (8.6%) | 45 (20.8%) | <0.001 | 462 (7.8%) | 42 (15.1%) | <0.001 |
| Medication used † | 2286 (46.8%) | 123 (56.9%) | 0.002 | 3370 (57.2%) | 198 (71.2%) | <0.001 |
| Prior heart disease ∗ | 493 (10.1%) | 42 (19.4%) | <0.001 | 1061 (18.0%) | 83 (29.9%) | <0.001 |
∗ Including coronary heart disease, arrhythmia and heart failure.
† Indicating any self-reported medication used in the past two weeks.
| Variables | Male | Female | ||||
|---|---|---|---|---|---|---|
| P | OR | 95% CI | P | OR | 95% CI | |
| Age (years) | <0.001 | 1.067 | 1.051-1.083 | <0.001 | 1.032 | 1.017-1.047 |
| Race (Han) | 0.465 | 1.280 | 0.659-2.485 | 0.610 | 0.848 | 0.450-1.597 |
| HR (beats/min) | 0.002 | 0.981 | 0.969-0.993 | <0.001 | 0.958 | 0.947-0.970 |
| Current smoker | 0.194 | 0.816 | 0.600-1.109 | 0.681 | 0.930 | 0.659-1.314 |
| Sleep duration (hours/day) | 0.656 | 1.020 | 0.936-1.110 | 0.853 | 0.993 | 0.922-1.069 |
| Activity level | 0.532 | |||||
| Low | 0.711 | 0.367 | ||||
| Moderate | 0.513 | 0.892 | 0.632-1.257 | 0.395 | 1.134 | 0.849-1.516 |
| High | 0.485 | 0.778 | 0.385-1.573 | 0.326 | 1.311 | 0.764-2.250 |
| Left ventricular ejection fraction | 0.847 | 1.154 | 0.269-4.944 | 0.541 | 0.662 | 0.176-2.484 |
| Left ventricular mass index (g/m 2 ) | 0.010 | 0.989 | 0.980-0.997 | 0.518 | 0.998 | 0.992-1.004 |
| Body mass index (kg/m 2 ) | 0.016 | 0.938 | 0.890-0.988 | 0.736 | 1.007 | 0.968-1.047 |
| Prior heart disease ∗ | 0.226 | 1.276 | 0.860-1.893 | 0.015 | 1.437 | 1.072-1.928 |
| Medication used † | 0.596 | 1.086 | 0.800-1.476 | 0.010 | 1.470 | 1.095-1.972 |
| Prior stroke | 0.005 | 1.747 | 1.184-2.577 | 0.038 | 1.485 | 1.021-2.158 |
| Serum magnesium (mmol/L) | 0.028 | 8.757 | 1.265-60.612 | 0.834 | 1.131 | 0.360-3.553 |
| Serum potassium (mmol/L) | <0.001 | 0.436 | 0.286-0.663 | <0.001 | 0.338 | 0.229-0.497 |
| Serum sodium (mmol/L) | 0.005 | 0.912 | 0.855-0.972 | 0.172 | 1.044 | 0.981-1.111 |
| Serum calcium (mmol/L) | 0.231 | 2.234 | 0.600-8.316 | 0.031 | 3.139 | 1.111-8.871 |
| Interventricular septal thickness (mm) | 0.001 | 7.050 | 2.139-23.241 | 0.564 | 1.330 | 0.505-3.500 |
| Left ventricular internal diastolic dimension (mm) | <0.001 | 2.937 | 1.918-4.498 | <0.001 | 2.186 | 1.515-3.155 |
| Posterior wall thickness (mm) | 0.011 | 3.827 | 1.360-10.772 | 0.271 | 1.727 | 0.652-4.574 |
| Alcohol consumption | ||||||
| None | 0.085 | 0.010 | ||||
| Moderate | 0.863 | 1.042 | 0.655-1.657 | 0.067 | 2.275 | 0.944-5.484 |
| Heavy | 0.031 | 1.431 | 1.033-1.982 | 0.012 | 2.344 | 1.202-4.571 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


