Obesity has been identified as a risk factor for cardiovascular disease and heart failure. However, data regarding the relation of body mass index (BMI) to outcome in patients with established heart failure are conflicting. We examined the risk of all-cause mortality and sudden cardiac death (SCD) in 1,231 patients after myocardial infarction with left ventricular dysfunction enrolled the Multicenter Automatic Defibrillator Implantation Trial-II (MADIT-II). Interaction-term analysis was used to assess the benefit of the implantable cardioverter–defibrillator (ICD) in upper (obese ≥30 kg/m 2 , n = 361) and lower (nonobese <30 kg/m 2 , n = 870) BMI categories. Mean BMI in the study population was 27.9 ± 5.1 kg/m 2 . In multivariate analysis, decreased BMI was shown to be independently associated with an increase in the risk of all-cause mortality (23% risk increase per 5-U BMI decreased, p = 0.009) and SCD (41% risk increase per 5-U BMI decrease, p = 0.01). Consistently, patients with BMI <30 kg/m 2 exhibited 46% (p = 0.03) and 76% (p = 0.04) increases in risk of all-cause mortality and SCD, respectively, compared to patients who had higher BMI values. The benefit of the ICD was pronounced in higher-risk patients with BMI <30 kg/m 2 (hazard ratio 0.68, p = 0.017) and maintained in the lower-risk subgroup of patients with BMI ≥30 kg/m 2 (hazard ratio 0.73, p = 0.32; p = 0.86 for ICD-by-BMI interaction). In conclusion, our findings suggest an independent inverse association between BMI values and risk of all-cause mortality and SCD in patients after myocardial infarction with left ventricular dysfunction enrolled in the MADIT-II trial.
Obesity has been shown to have adverse effects on several coronary artery disease risk factors, including hypertension, left ventricular (LV) hypertrophy, dyslipidemia, and the development of diabetes mellitus and the metabolic syndrome. Accordingly, large cohort studies have shown that increased body mass index (BMI) is an independent risk factor for the development of coronary artery disease and heart failure (HF). However, data regarding the association between increased BMI and outcome in patients with established HF are conflicting. Gustafsson et al showed that in patients with LV systolic dysfunction, obesity was associated with increased risk. In contrast, several recent studies have suggested that in patients with HF there is an inverse association between BMI and subsequent prognosis. Furthermore, low BMI has been shown to be an important predictor of mortality in patients with an implantable cardioverter–defibrillator (ICD). Thus, it is possible that patients with HF and lower BMIs may have increased risk for sudden cardiac death (SCD), and that this relation may affect the benefit of the ICD in this population. The present study was designed to (1) evaluate the relation between BMI and all-cause mortality and SCD in patients with ischemic LV dysfunction enrolled in the Multicenter Automatic Defibrillator Implantation Trial-II (MADIT-II) and (2) assess the effect of BMI on the benefit of primary ICD therapy.
Methods
The design and results of MADIT-II have been reported previously. Briefly, 1,232 patients with documented previous myocardial infarction and an LV ejection fraction ≤30% were randomized to receive a prophylactic ICD or conventional medical therapy in a 3:2 ratio. Screened patients were excluded from enrollment if they had class IV HF, coronary revascularization within the previous 3 months, elapsed interval from most recent myocardial infarction of <1 month, end-stage renal disease, or any other advanced medical co-morbidity. After a mean follow-up of 20 months, ICD therapy was shown to be associated with a significant 31% decrease in the risk of death compared to non-ICD conventional medical therapy.
Weight and height measurements were obtained from 1,231 study patients (99.9%) on enrollment in the trial. BMI was calculated as weight in kilograms divided by the square of the height in meters and was assessed as (1) a continuous measure (per 5-U decrease) and (2) a categorical variable, dichotomized in the primary analysis according to the clinical definition of obesity (BMI ≥30 kg/m 2 ). In a secondary analysis, patients in the nonobese category were further divided into overweight (25 to 29 kg/m 2 ) and normal/underweight (<25 kg/m 2 ) subcategories. The prespecified outcome measurements of the present study included all-cause mortality and SCD. A modified Hinkle-Thaler system was used to classify deaths as described previously.
We evaluated (1) the relation between BMI and risk of all-cause mortality and SCD and (2) the survival benefit associated with ICD therapy as a function of BMI category.
Characteristics of study patients by BMI category were compared by Wilcoxon rank-sum, chi-square, or Fisher’s exact test, as appropriate. Probability of all-cause mortality and SCD by BMI category and by treatment group in each BMI category was graphically displayed according to the Kaplan-Meier method, with comparison of cumulative events by log-rank test. Cox proportional hazards regression model was used to evaluate the independent contribution of baseline clinical factors to the development of end points. Baseline variables that had differences between the 2 BMI categories, using a p value <0.10, were evaluated in the proportional hazards stepwise selection model. Covariates with a p value <0.05 in the proportional hazards model were included in the final model. In an alternative analysis, gender, New York Heart Association class ≥III, QRS duration >0.12 second, diabetes mellitus, and medical therapy with β blockers, angiotensin-converting enzyme inhibitors, and lipid-lowering agents were forced into multivariate models as additional covariates. Effect of BMI on outcome measurements of all-cause mortality and SCD was assessed in the total population, and the survival benefit of the ICD in each BMI category was assessed by including a treatment-by-BMI interaction term in multivariate models. All p values were 2-sided, and a p value <0.05 was considered statistically significant. Analyses were performed using SAS 9.20 (SAS Institute, Cary, North Carolina).
Results
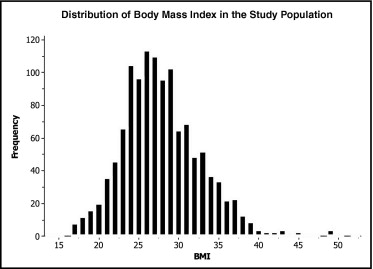
Mean BMI of study patients was 27.9 ± 5.1 kg/m 2 (median 27.3, interquartile range 24.4 to 30.8) and was normally distributed ( Figure 1 ). Patients in the lower (nonobese) BMI category had BMIs mostly in the normal to overweight range (mean 25.4 ± 2.9 kg/m 2 ), whereas patients in the upper (obese) BMI category had mean BMIs of 34.1 ± 3.8 kg/m 2 . Table 1 presents baseline characteristics of study patients by BMI category. Compared to nonobese patients, patients with a BMI ≥30 kg/m 2 were younger and had a higher ejection fraction and a higher frequency of hypertension, diabetes mellitus, smoking, and faster baseline heart rates. In addition, medical therapy with lipid-lowering agents was employed more frequently in obese patients.
| Characteristic | BMI (kg/m 2 ) | p Value | |
|---|---|---|---|
| <30 | ≥30 | ||
| (n = 870) | (n = 361) | ||
| Body mass index (kg/m 2 ) | 25.4 ± 2.9 | 34.1 ± 3.8 | NA |
| Age >65 years | 60% | 40% | <0.001 |
| Women | 14% | 19% | 0.03 |
| New York Heart Association class ≥III ⁎ | 28% | 30% | 0.44 |
| Angina pectoris functional class ≥2 | 27% | 30% | 0.24 |
| Hypertension | 51% | 60% | 0.002 |
| Diabetes mellitus | 30% | 47% | <0.001 |
| Previous coronary artery bypass grafting | 58% | 57% | 0.80 |
| Left bundle branch block | 19% | 17% | 0.45 |
| QRS duration >0.12 second | 37% | 34% | 0.32 |
| Ejection fraction <25% | 50% | 42% | 0.02 |
| Atrial fibrillation | 28% | 25% | 0.38 |
| Cigarette smoking anytime | 78% | 86% | 0.001 |
| Systolic blood pressure ≥130 mm Hg | 34% | 35% | 0.67 |
| Diastolic blood pressure ≤80 mm Hg | 75% | 69% | 0.04 |
| Heart rate ≥80 beats per min | 27% | 35% | 0.006 |
| Serum urea nitrogen >25 mg/dl | 32% | 27% | 0.08 |
| Medical therapy | |||
| Angiotensin-converting enzyme inhibitors | 78% | 76% | 0.26 |
| Digitalis | 59% | 58% | 0.76 |
| β blockers | 60% | 68% | 0.01 |
| Amiodarone | 12% | 15% | 0.15 |
| Lipid-lowering agents † | 63% | 71% | 0.007 |
| Diuretics | 73% | 79% | 0.02 |
⁎ New York Heart Association and angina pectoris functional class represent the highest class during the 3 months before enrollment.
Kaplan-Meier survival analysis ( Figure 2 ) demonstrated that 2-year event rates were significantly higher in patients with BMI <30 kg/m 2 compared to those who exhibited higher BMI values (all-cause mortality 24% vs 14%, respectively, p = 0.003; SCD 11% vs 4%, respectively, p = 0.007).
Clinical covariates shown to be independently associated with an increase in the risk of all-cause mortality and SCD in the Cox regression analysis included older age, a lower ejection fraction, increased serum urea nitrogen levels, fast baseline heart rate, and cigarette smoking ( Table 2 ). Consistent with Kaplan-Meier results, multivariate analysis demonstrated that BMI was inversely related to the 2 outcome measurements; when assessed as a continuous variable, each 5-U decrement in BMI was independently associated with a significant 23% (p = 0.009) increase in the risk of all-cause mortality and a 41% (p = 0.01) increase in the risk of SCD. Similarly, when BMI was assessed as a categorical covariate, patients with a BMI <30 kg/m 2 were shown to have a 46% (p = 0.03) increase in the risk of all-cause mortality and a 76% (p = 0.04) increase in the risk of SCD compared to patients who had higher BMI values. Interaction-term analysis demonstrated that the effect of BMI on outcomes was not significantly different between patients with and without ICD (all BMI-by-treatment interaction terms >0.20). A secondary analysis, in which patients in the nonobese category were further divided into overweight (BMI 25 to 29 kg/m 2 ) and normal/underweight (<25 kg/m 2 ) subcategories, consistently demonstrated increasing rates of all-cause mortality and SCD with decreasing BMI values ( Figure 3 ). Thus, in multivariate analysis patients with BMI <25 kg/m 2 had significant 56% (p = 0.02) and 99% (p = 0.04) increases in the risk of all-cause mortality and SCD, respectively, compared to patients with BMI ≥30 kg/m 2 , and patients with BMI 25 to 29 kg/m 2 had an intermediate risk increase (38%, p = 0.09, and 59%, p = 0.16, respectively; p for trend = 0.04 for 2 end points).
| Variable | All-Cause Mortality | Sudden Cardiac Death | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Defibrillator versus conventional | 0.69 (0.52–0.92) | 0.001 | 0.36 (0.23–0.58) | <0.0001 |
| Serum urea nitrogen >25 mg/dl | 2.26 (1.69–3.03) | <0.0001 | 1.98 (1.24–3.16) | 0.004 |
| Age >65 years | 1.79 (1.30–2.46) | 0.0003 | 1.92 (1.14–3.24) | 0.01 |
| Ejection fraction <25% | 1.55 (1.17–2.07) | 0.003 | 1.930 (1.21–3.09) | 0.006 |
| Heart rate ≥80 beats/min | 1.43 (1.07–1.91) | 0.01 | 2.08 (1.33–3.27) | 0.001 |
| Smoker | 1.51 (1.03–2.20) | 0.04 | 1.35 (0.73–2.48) | 0.34 |
| Body mass index (kg/m 2 ) † | ||||
| A. Continuous: per 5-U decrease | 1.23 (1.05–1.43) | 0.009 | 1.41 (1.09–1.83) | 0.01 |
| B. Categorized: <30 versus ≥30 | 1.46 (1.03–2.07) | 0.03 | 1.76 (1.01–3.24) | 0.04 |
⁎ Hazard ratios for variables were obtained from multivariate models that adjusted for body mass index as a continuous measurement; similar results regarding the association of body mass index with the 2 outcome measurements were obtained in multivariate models that included further adjustment for gender, New York Heart Association class, QRS ≥120 ms, diabetes mellitus, and treatment with β blockers, angiotensin-converting enzyme-inhibitors, and lipid-lowering agents.
† There was no significant difference in the effect of body mass index on outcome between the 2 treatment groups (all p values for treatment-by-body mass index interaction terms >0.20); similar hazard ratios for the effect of body mass index were obtained in models that included further adjustment for gender, New York Heart Association class, QRS ≥120 ms, diabetes mellitus, and treatment with β blockers, angiotensin-converting enzyme-inhibitors, and lipid-lowering therapies.




