The determinants and prognostic value of recurrent myocardial infarction (MI) in a contemporary cohort of ST-segment elevation MI patients treated with primary percutaneous coronary intervention (PPCI) and stenting are currently unknown. We investigated the predictors and prognostic impact of recurrent MI on subsequent clinical outcome in 1,700 ST-segment elevation MI patients treated with PPCI and stenting between January 1, 2003, and July 31, 2008. Two hundred forty patients had a recurrent MI during a median follow-up of 4 years and 7 months (Kaplan Meier estimate 21.2%). By multivariable analysis, recurrent MI was associated with a higher risk of subsequent cardiac mortality (hazard ratio [HR] 6.86, 95% confidence interval [CI] 4.24 to 8.72), noncardiac mortality (HR 2.02, 95% CI 1.10 to 3.69), stroke (HR 3.68, 95% CI 2.02 to 6.72), and Global Use of Strategies to Open Occluded Coronary Arteries criteria severe or moderate bleeding (HR 3.17, 95% CI 1.79 to 5.60). Early recurrent MI (within 1 day of the initial PPCI) was associated with higher unadjusted cardiac mortality rates (64.4%) compared with recurrent MIs occurring ≥1 day after PPCI. However, after multivariable adjustment, late recurrent MI (occurring >1 year after PPCI) was associated with the highest risk of subsequent cardiac mortality (HR 7.98, 95% CI 5.05 to 12.6). The risk of cardiac death was irrespective of the presence of persistent ST-segment elevation during the recurrent MI. In conclusion, recurrent MI after PPCI remains a relatively common complication in contemporary practice and confers a significantly increased risk of death, stroke, and bleeding.
Recurrent myocardial infarction (MI) after ST-segment elevation MI (STEMI) is associated with increased morbidity and mortality. Fortunately, however, mortality rates after recurrent MI have recently been shown to be declining over the past 2 decades. The introduction of new high-sensitive biomarker assays has enabled the detection of recurrent MIs with smaller amounts of myocardial necrosis, which may explain in part the reduced mortality after recurrent MI. Moreover, current treatment strategies, including advances in revascularization therapy have decreased mortality after recurrent MI. Routine stenting of the culprit lesion is recommended over balloon angioplasty during primary percutaneous coronary intervention (PPCI) to prevent coronary restenosis. Although treatment with bare-metal or drug-eluting stents has not resulted in reduced rates of recurrent MI after PPCI, it is possible that clinical and angiographic correlates of recurrent MI have changed with the introduction of coronary stents. These correlates have not been well-described in a contemporary cohort of STEMI patients treated with coronary stenting and double or triple antithrombotic therapy, however. Therefore, the aim of this study was to investigate the predictors and clinical outcome after recurrent MI within 5 years’ follow-up after PPCI in STEMI patients treated with double antiplatelet therapy.
Methods
The data analyzed in this study were obtained from STEMI patients who were accepted for PPCI at the Academic Medical Center, University of Amsterdam, between January 1, 2003, and July 31, 2008. The study complied with the Declaration of Helsinki, and the local ethics committee approved the study protocol. In general, patients qualified for PPCI if they had typical ischemic chest pain and at least 1-mm ST-segment elevation in ≥2 contiguous leads, a new left bundle branch block, or a true posterior MI. The PPCI and adjunctive pharmacologic treatment were performed according to American College of Cardiology, American Heart Association, and European Society of Cardiology guidelines. Patients received a standard 300- to 600-mg loading dose of clopidogrel. If a coronary stent was implanted, clopidogrel was prescribed for ≥1 month to patients with a bare metal stent and for 6 to 12 months to patients with a dug-eluting stent. Patients were routinely pretreated with 300 mg aspirin and 5,000 IU unfractionated heparin. An additional heparin bolus was administered at the catheterization laboratory if necessary to achieve a targeted activated clotting time of 300 seconds followed by an infusion of 12 U/kg/h with titration to achieve a target activated partial thromboplastin time (aPTT) of 1.5 to 2.0 times the control. Glycoprotein IIb/IIIa inhibitors were used at in a bailout setting at the discretion of the operator.
Procedural and angiographic data were prospectively collected by interventional cardiologists and specialized nurses in a dedicated database. Chart review for consecutive STEMI patients with available aPTT measurements was performed in the context of a study designed to investigate the relationship between aPTT and clinical outcome in STEMI patients treated with PPCI. A detailed description of the study protocol has been previously published. We obtained clinical history, detailed information on peri-procedural treatment and follow-up of clinical outcome, including recurrent MI, stroke, stent thrombosis, and bleeding by reviewing inpatient and outpatient charts in the tertiary percutaneous coronary intervention (PCI) center and referring hospitals between 2011 and 2012. For every patient, we systematically checked inpatient charts of every hospital admission for the occurrence of the aforementioned clinical events. Follow-up of clinical events was censored at the actual date of chart review. Patients whose whereabouts could not be traced were considered lost to follow-up from the date of last known medical contact. Follow-up information regarding vital status was obtained from computerized, long-term mortality records from the National Death Index between January 1, 2012, and April 30, 2012. If a patient could not be identified in these records (e.g., foreign patients), censoring was at the date of last contact.
The study cohort consisted of all STEMI patients included in our study database, who were alive at the end of the procedure. We excluded patients in whom no coronary stent was implanted during the initial PPCI. A recurrent MI was defined according to the Academic Research Consortium criteria for MI (a detailed description can be found in the online Supplemental Material ).
Procedure (PCI or coronary artery bypass graft [CABG])-related MIs during follow-up were not considered recurrent MIs in the current study. Only the first recurrent MI was taken into consideration and categorized as STEMI or non-STEMI (NSTEMI). STEMI was defined as an MI characterized by new ST-segment elevation of ≥1 mm in 2 contiguous leads, a new left bundle branch block, or a true posterior infarction. NSTEMI was defined as a recurrent MI not meeting the criteria for STEMI.
We investigated the impact of recurrent MI on subsequent clinical outcome including, all-cause, cardiac and noncardiac mortality, ischemic and hemorrhagic stroke, and bleeding. Cardiac death was defined according to the Academic Research Consortium criteria. Bleeding complications were defined according to the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries (GUSTO) criteria. Stroke was defined as an irreversible neurologic deficit, as classified by the treating neurologist, on the basis of supporting information, including brain images and neurologic evaluation.
Normally distributed continuous variables were reported as the mean with SD and compared with Student’s t test, skewed distributed variables were presented as the median with interquartile range and compared with the Wilcoxon rank-sum test. Categorical variables were presented as proportions and compared with the chi-square test. Event rates of recurrent MI and cardiac death were estimated using Kaplan-Meier analyses. We used stepwise backward selection Cox proportional hazards models to determine independent predictors of recurrent MI. Entry and exit criteria were set at the p <0.05 and p <0.1 level, respectively.
We investigated predictors of recurrent MI occurring (1) in the first 30 days, (2) between 30 days and 1 year, and (3) beyond 1 year. Patients who survived for 30 days without recurrent MI were included in the landmark analyses for recurrent MIs after 30 days, and patients who survived for 1 year without recurrent MI were included in the landmark analyses recurrent MIs occurring after 1 year.
The relation between the occurrence of recurrent MI and subsequent clinical outcomes (including cardiac, noncardiac, and all-cause mortality, stroke, and GUSTO severe and moderate bleeding) was investigated by inserting the occurrence of a recurrent MI as a time-dependent variable in 2 sets of Cox proportional hazards models for each outcome: unadjusted models and models adjusted for relevant predictors of the clinical outcomes. Relevant predictors were determined by performing backward selection stepwise Cox models. To investigate if the magnitude of risk of subsequent cardiac mortality was dependent on the presence of ST-segment elevation during recurrent MI, we performed additional univariate and multivariate time-dependent Cox models simultaneously including recurrent STEMI and NSTEMI as time-dependent covariates.
To investigate if the timing of the recurrent MI in relation to the index MI had significant impact on the prognostic value for subsequent cardiac mortality, we developed additional time dependent Cox models in which the time elapsed after the index MI was stratified in the time intervals of 0 to 1 days, 2 to 7 days, 8 to 30 days, 31 to 365 days, and beyond 365 days. For each time interval, the subsequent cardiac mortality was estimated using Kaplan-Meier analyses, and hazard ratios were calculated using Cox models.
Data were complete for all outcomes and for 26 of 40 covariates. Table 1 in the online Supplemental Material presents the percentage of missingness for all covariates. Missing patient-level covariates were assumed to be missing at random and were imputed with the use of multiple imputations. The imputation procedure and subsequent Cox proportional hazards regression estimation were performed according to Rubin’s protocol. Pooled estimates of the imputed data were considered primary analyses. As a sensitivity analysis, we repeated all analyses in the original dataset (complete case analysis). All analyses were performed with Statistical Package for Social Sciences software (SPSS version 19.0, Chicago, Illinois).
| Variable | Recurrent MI | p Value | |
|---|---|---|---|
| Yes (n = 240) | No (n = 1,460) | ||
| Men | 166 (69.2%) | 1,037 (71.0%) | 0.56 |
| Age (yrs), median (IQR) | 63 (53–76) | 62 (52–71) | 0.075 |
| BMI, median (IQR) | 26.3 (24.0–29.1) | 26.1 (24.2–28.7) | 0.26 |
| History of | |||
| Diabetes mellitus | 55 (22.9%) | 171 (11.7%) | <0.001 |
| IDDM | 17 (7.1%) | 43 (2.9%) | |
| NIDDM | 38 (15.8%) | 128 (8.8%) | |
| Hypertension | 108 (45.0%) | 527 (36.1%) | 0.008 |
| Dyslipidemia | 66 (27.5%) | 309 (21.2%) | 0.028 |
| Previous stroke or TIA | 16 (6.7%) | 91 (6.2%) | 0.80 |
| Peripheral artery disease | 23 (9.6%) | 75 (5.1%) | 0.006 |
| Preexistent malignant disease | 18 (7.5%) | 110 (7.5%) | 0.99 |
| Recent surgery (<10 days) | 1 (0.4%) | 19 (1.3%) | 0.24 |
| Bleeding | 14 (5.8%) | 48 (3.3%) | 0.051 |
| Current smoking | 105 (43.8%) | 670 (45.9%) | 0.54 |
| Previous MI | 43 (17.9%) | 139 (9.5%) | <0.001 |
| Previous PCI | 27 (11.3%) | 101 (6.9%) | 0.018 |
| Previous CABG | 9 (3.8%) | 21 (1.4%) | 0.012 |
| Family history CAD | 95 (39.7%) | 552 (37.8%) | 0.60 |
| Laboratory values | |||
| Hemoglobin (mmol/L), median (IQR) | 8.8 (8.0–9.4) | 8.9 (8.2–9.5) | 0.25 |
| Leucocyte count (×10 9 /L) | 11.2 (9.1–14.3) | 11.3 (8.9–14.4) | 0.77 |
| Creatinine clearance (ml/min/1.73 m 2 ), median (IQR) | 84.2 (62.8–119.5) | 93.0 (70.0–118) | 0.064 |
| Thrombocyte count (×10 9 /L) | 0.55 | ||
| <150 | 7/239 (2.9%) | 58/1,444 (4.0%) | — |
| 150–400 | 226/239 (94.6%) | 1,337/1,444 (92.5%) | — |
| >400 | 6/239 (2.5%) | 49/1,444 (3.4%) | — |
Results
Of the 2,009 STEMI patients recorded in our database, 2,002 were alive at the end of the procedure. Eighty patients did not undergo PCI, and 222 were treated with balloon angioplasty only; 1,700 were treated with a stent during the index PPCI and comprise the study cohort for the present analysis. Figure 1 displays the Kaplan-Meier curve for the occurrence of recurrent MI after STEMI. In total, there were 240 recurrent MIs during a median follow-up of 5 years and 5 months (interquartile range 4.2 to 6.8 years), of which 46 (19.2%) were caused by a definite stent thrombosis of ≥1 of the stents implanted during the index PCI. Fifty-seven (3.5%) patients suffered a recurrent MI within 30 days. Of these, 30 presented with persistent ST-segment elevation, and 27 presented without persistent ST-segment elevation. Twenty-two of the 57 (38.6%) recurrent MIs within 30 days were caused by stent thrombosis. Of the 240 patients who suffered a recurrent MI, 88 suffered a recurrent MI with persistent ST-segment elevation (36.7%), and 152 suffered a recurrent MI without persistent ST-segment elevation (63.3%).
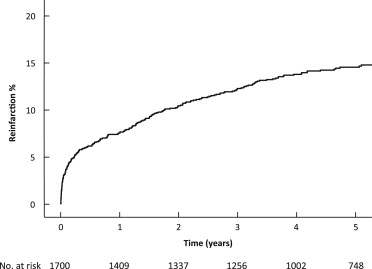
Tables 1 and 2 display baseline clinical, procedural, and angiographic characteristics of patients with and without recurrent MI during follow-up. Table 3 displays the independent predictors of a recurrent MI occurring in the first 30 days, between day 31 and 1 year, and beyond 1 year. Supplementary Tables 2 through 4 of the Online Supplement display predictors of recurrent MI with and without persistent ST-segment elevation and predictors of recurrent MI caused by stent thrombosis. None of the factors associated with recurrent STEMI were predictive of recurrent NSTEMI. A history of previous MI, peripheral artery disease, dyslipidemia, previous CABG, anterior infarction, coronary calcification, young age, and a 300-mg clopidogrel loading dose (rather than 600 mg) were predictive of recurrent STEMI. By contrast, body mass index, diabetes, a history of bleeding, lower creatinine clearance, multivessel disease, total ischemic time, and ostial lesions were associated with recurrent NSTEMI.
| Variable | Recurrent MI | p Value | |
|---|---|---|---|
| Yes (n = 240) | No (n = 1,460) | ||
| Total ischemic time (min), Median (IQR) | 190 (128–270) | 177 (128–268) | 0.48 |
| Peak CK-MB release (mmol/L), Median (IQR) | 239 (99.2–469) | 230 (103–433) | 0.35 |
| Shock | 18/239 (7.4%) | 108/1,453 (7.5%) | 0.96 |
| Loading dose clopidogrel | 230/235 (97.9%) | 1,408/1,422 (99.0%) | 0.016 |
| 300 mg | 170/235 (72.3%) | 913/1,422 (64.2%) | |
| 600 mg | 60/235 (25.5%) | 487/1,422 (34.2%) | |
| Other | 0/235 (0.0%) | 8/1,422 (0.6%) | |
| Glycoprotein IIb/IIIa inhibitor | 86 (35.8%) | 395 (27.1%) | 0.005 |
| IRA | 0.46 | ||
| RCA or LCx | 12 (53.8%) | 822 (56.3%) | |
| LAD or LM | 111 (46.3%) | 638 (43.7%) | |
| Preprocedural TIMI flow in IRA | 0.71 | ||
| 0–1 | 163/225 (72.4%) | 983/1,380 (71.2%) | |
| 2–3 | 62/225 (27.6%) | 397/1,380 (28.8%) | |
| Postprocedural TIMI flow in IRA | 0.054 | ||
| 0–1 | 4/236 (1.7%) | 8/1,444 (0.6%) | |
| 2–3 | 232/236 (98.3%) | 1436/1,444 (99.4%) | |
| Multivessel disease | 114/236 (48.3%) | 499/1,422 (34.8%) | <0.001 |
| Chronic total occlusion | 46/236 (19.5%) | 179/1,432 (12.5%) | 0.004 |
| Intracoronary thrombus | 149 (62.1%) | 860 (58.9%) | 0.35 |
| Ostial lesion | 41 (17.1%) | 171 (11.7%) | 0.020 |
| Calcification | 72 (30.0%) | 281 (19.2%) | <0.001 |
| Preexisting dissection | 49 (20.4%) | 285 (19.5%) | 0.75 |
| Amount of lesions treated | 0.089 | ||
| 1 | 231 (96.3%) | 1,432 (98.1%) | |
| 2 | 9 (3.8%) | 25 (1.7%) | |
| 3 | 0 (0.0%) | 3 (0.2%) | |
| Stent | 0.044 | ||
| Bare-metal stent ∗ | 229 (95.4%) | 1,426 (97.7%) | |
| Drug-eluting stent | 11 (4.6%) | 34 (2.3%) | |
| Amount of stents | 0.36 | ||
| 1 | 192 (80.0%) | 1,224 (83.8%) | |
| 2 | 41 (17.1%) | 201 (13.8%) | |
| 3 | 7 (2.9%) | 31 (2.1%) | |
| 4 | 0 (0.0%) | 4 (0.3%) | |
| Vessel diameter (mm), median (IQR) | 3.5 (3.0–3.5) | 3.5 (3.0–3.5) | 0.001 |
| Stent length (mm), median (IQR) | 20 (15–26) | 18 (15–24) | 0.19 |
∗ Includes 10 patients who were treated with an endothelial progenitor cell–capturing stent.
| HR | 95% CI | p Value | |
|---|---|---|---|
| 30 day recurrent MI | |||
| Multivessel disease without CTO | 2.98 | 1.62–5.49 | <0.001 |
| Multivessel disease with CTO | 2.91 | 1.43–5.90 | 0.003 |
| Calcification of the culprit vessel | 1.74 | 1.19–3.55 | 0.052 |
| History of stroke or TIA | 2.17 | 1.02–4.63 | 0.045 |
| Family history of CAD | 1.64 | 0.97–2.78 | 0.066 |
| Intracoronary thrombus | 1.69 | 0.95–3.00 | 0.076 |
| Vessel diameter (per 1-mm decrease) | 1.96 | 1.08–3.57 | 0.028 |
| Total ischemic time (per 30 min) | 1.03 | 1.01–1.04 | 0.003 |
| Recurrent MI between 31 days and 1 yr | |||
| Diabetes mellitus | |||
| NIDDM | 2.47 | 1.32–4.61 | 0.005 |
| IDDM | 3.63 | 1.64–8.00 | 0.001 |
| Peripheral artery disease | 3.83 | 1.94–7.58 | <0.001 |
| Ostial lesion | 2.68 | 1.53–4.71 | 0.001 |
| Previous MI | 2.20 | 1.20–4.03 | 0.011 |
| Vessel diameter (per 1-mm decrease) | 1.60 | 0.95–2.68 | 0.075 |
| Recurrent MI after 1 yr | |||
| Diabetes mellitus | |||
| NIDDM | 1.92 | 1.49–2.47 | 0.009 |
| IDDM | 1.49 | 0.94–2.37 | 0.50 |
| Previous MI | 1.70 | 1.33–2.18 | 0.030 |
| Calcification of the culprit vessel | 1.53 | 1.25–1.89 | 0.039 |
| Stent length (per 1-mm increase) | 1.02 | 1.02–1.03 | 0.001 |
| All recurrent MIs | |||
| Diabetes mellitus | |||
| NIDDM | 1.69 | 1.18–2.41 | 0.004 |
| IDDM | 2.29 | 1.38–3.81 | 0.001 |
| History of bleeding | 1.89 | 1.09–3.27 | 0.024 |
| Peripheral vascular disease | 1.89 | 1.22–2.92 | 0.004 |
| GP IIb–IIIa inhibitors | 1.53 | 1.17–2.00 | 0.002 |
| Total ischemic time (per 30 min) | 1.02 | 1.00–1.03 | 0.026 |
| Multivessel disease without CTO | 1.52 | 1.12–2.06 | 0.007 |
| Multivessel disease with CTO | 1.61 | 1.13–2.31 | 0.009 |
| Previous MI | 1.47 | 1.03–2.10 | 0.032 |
| Calcification of the culprit vessel | 1.55 | 1.16–2.06 | 0.003 |
| Vessel diameter (per 1-mm decrease) | 1.47 | 1.12–1.94 | 0.006 |
| Total ischemic time (per 30 min) | 1.01 | 1.00–1.03 | 0.042 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


