Fig. 1.1
3D echocardiography by ECG-gated rotational device. The upper figures show how to obtain the actual image by using ECG-gated rotational device. The probe is placed on the patient from subxiphoid window. The lower right figure shows the 3D echocardiography in a case with complete atrioventricular septal defect by the system that rotates by 2 s and collects the images of every heartbeat to reconstruct. RA right atrium, RV right ventricle, ASD(I) primum atrial septal defect, CAVV common atrioventricular valve
Regarding children, moreover, it was problematic in terms of quality of images even after collecting volume data over time and reconstructing based on them because of their fast heat rate and difficulty of breath-holding compared with adults. In other words, the stitches caused by heartbeat synchronization and the gaps caused by respiratory fluctuation can affect the quality of the images significantly. In addition, enormous amount of time was necessary for off-line image analysis (the right lower panel in Fig. 1.1). Therefore, 3D echocardiography was rarely used for the diagnosis of a complicated form of congenital heart disease in the actual clinical practice.
However, recently, high-resolution volume datasets have to be collected in any direction from a single to a few heartbeat datasets, when using the real-time 3D echocardiography. The development of the high-frequency 3D probe for children and the improvements of image quality, even if, by using the low-frequency probe have contributed to the issue. In addition, since the performance improvement of the analysis workstation has enabled the volume data analysis in the extremely short period of time, it has become possible to visualize the optimum cross section as well.
The following are the good examples of clinical applications of the real-time 3DE for congenital heart diseases.
1.
As the guide for surgical repair: Intracardiac route creation via ventricular septal defect (VSD) with double-outlet right ventricle and valvuloplasty for the complicated atrioventricular valve insufficiency
2.
As the guide and monitoring for the percutaneous catheter closure of atrial septal defect or ventricular septal defect
3.
As the 3D functional analysis of volume and wall motion both in the left and right ventricle and quantitative evaluation of the dynamic morphological of the atrioventricular valve leaflets in congenital heart disease
Once the methodology is established, when it comes to children whose echo windows are easy to obtain and have relatively clear images, it is evident that the real-time 3D echocardiography would become even more useful for the understanding of the complicated anatomical structure than the adult’s cardiovascular diseases.
In this chapter, I would like to discuss mainly how to use the transpericardial real-time 3D echocardiography as a guide for surgical repair in the actual clinical practice. In addition, I also would like to outline the usage of the transesophageal real-time 3D echocardiography as the guide and monitoring for the percutaneous catheter closure.
1.2 3D Display of the Intracardiac Structure
Roll as a tool for detailed diagnosis and a guide for surgery.
Since 3D display of the intracardiac structure enables to understand the anatomically abnormal findings, it is an extremely useful method for considering the hemodynamic status and the operative procedure for intracardiac surgical repair [2]. It can be utilized for various surgeries such as closure operation for the multiple or complicated VSDs, creation of the intracardiac route via ventricular defect with double-outlet right ventricle and transposition of the great arteries, release for stenotic or obstructive lesions such as the left of right ventricular outflow tract and the pulmonary vein, and the atrioventricular valvuloplasty for congenital heart disease. One major point of the 3D display as a guide of the congenital heart disease is how to present it to surgeons. Creating the images from the surgeon’s standing point, i.e., surgeon’s view, would serve as the base of communication between cardiologists and cardiac surgeons and also help surgeons understand with ease.
1.2.1 Transpericardial 3D Echocardiography
Although transthoracic 3D echocardiography has a certain level of diagnostic accuracy, we perform intraoperative transpericardial 3D echography with the aim to construct the good quality of images that would have more diagnostic accuracy and would be helpful for surgery [3]. In adult patients having severe mitral regurgitation, it is difficult to perform the detailed guide for mitral valvuloplasty by using transthoracic 3D echocardiography, while the transesophageal 3D echocardiography is better than that. Since the body weight of the most children with congenital heart disease, who undergo intracardiac repair that needs a cardiopulmonary bypass, is less than 15 kg, it is impossible to insert the probe of the current transesophageal 3D echocardiography. For the children for whom the transesophageal probe cannot be used, the transpericardial approach is probably the best 3D echocardiography currently because it produces best images and high sensitivity. Specifically, the images can be obtained by applying the 3D probe directly on the pericardium or heart under thoracotomy. Volume data will be obtained at the full-volume mode using 3D probe by temporarily shutting off the artificial respirator only when the breathing movement is influential. Of course, clearer images with higher resolution and better S/N ratio than transthoracic 3D echocardiography will be obtained (Fig. 1.2).

Fig. 1.2
The actual procedure of 3D pericardial echocardiography volume dataset can be acquired to put the 3D probe directly on the pericardium under thoracotomy, using 3D probe by temporarily shutting off the artificial respirator only when the breathing movement is influential. Then good quality of reconstructed images with higher resolution and better S/N ratio compared to the images by transthoracic 3D echocardiography can be obtained
1.2.2 Accommodation of Images
Collecting high-quality volume data is the key to obtain the 3D images with higher quality. The first step is to select the appropriate probe. For the children with body weight less than 20 kg, it might be better to use a 3D probe with as high frequency as possible (more than 7 MHz). For those with body more than that, a 3D probe with 5 MHz frequency should be used. First, capture the 2D images. Then, decide where to put the center for collecting the 3D images. It is important to put the probe from the window that can best visualize the target lesion. It might be better to confirm whether the whole target sites are visualized properly by the biplane mode, the multi-slice mode, and tilting the probe. Adjustment such as “gain” and “dynamic range” should be controlled on the 2D images. One of the keys to success is to put the probe on the pericardium tightly.
1.2.3 Volume Data Collection
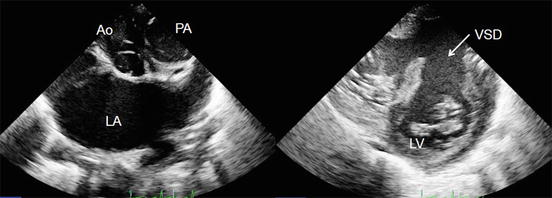
After adjusting the 2D images, start to work on the 3D image collection. It is important to capture the images considering what kind of 3D images you would like to compose at the end. For example, suppose that the disease is VSD. Figure 1.3 is the schema to observe the interventricular septum that was cut out from the right atrium and the right ventricular free wall at the frontal plane. Based on the anatomical relationship among the defect, the tricuspid valve, the pulmonary valve, and the aortic valve, it can be classified into perimembranous VSD, doubly committed VSD, trabecular VSD, and inflow septal VSD. If the 3D volume data is cut similarly as in this figure, it would help to determine what kind of approach would be appropriate to close the VSD, or whether it should be performed from the pulmonary artery or the tricuspid valve, or under which leaflet of tricuspid valve the defect exists. It might be better to understand the anatomical knowledge and representative operative procedures regarding the VSD before data acquisition. Figure 1.4 shows the comparison between the transpericardial 3D image with the VSD and the surgical finding. Similarly as in the surgical findings, the defect is seen in perimembranous portion, nearly the upper part of the septal leaflet of the tricuspid valve. In addition, the chorda tendinea of the tricuspid valve appears to cross over the defect, which can be clearly confirmed by the 3D echocardiography. To close the defect, it used to be necessary to make a resection avoiding the chorda tendinea [3]. In the assessment of the double-outlet right ventricle and the VSD that is porous and more complicated, the positional relationship among the pulmonary valve, the aortic valve, the tricuspid valve, and the abnormal chorda tendinea in addition to the size or the number becomes even more important in order to determine the method of closing the defect and forming the intracardiac route. Therefore, the volume data should be collected so that not only the VSD but also all the surrounding large vessels and atrioventricular valves would be included. The volume rate at the time of collecting should be over 40 Hz when the heart rate is around 100 bpm. Since a wide angle becomes necessary due to the necessity of including the surrounding structures, capturing in the full-volume mode integrated with ECG-synchronized multiple slices (heartbeats) could maintain the beamline density higher than capturing the single heartbeat with low volume rate.
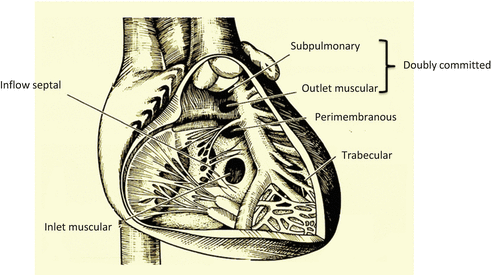
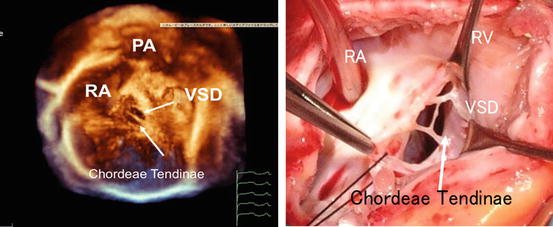

Fig. 1.3
Type of the ventricular septal defects

Fig. 1.4
The comparison between the transpericardial 3D image with the ventricular septal defect and the surgical finding. RA right atrium, PA pulmonary artery, RV right ventricle, VSD ventricular septal defect
1.2.4 Cropping
The next step is to create the images viewed from the surgeon’s position, so-called surgeon’s view. To that end, it is necessary to understand the anatomical features of the disease and representative operative procedures and keep in mind how to proceed with the cropping to make the cut-plane. Here is an example of the actual cropping case of the double-outlet right ventricle. Figure 1.5 shows a case with VSD in double-outlet right ventricle. The 2D echography reveals that the aorta is located in the right posterior and the pulmonary artery in the left anterior while the VSD exists subpulmonary. In Fig. 1.6, the 3D image by cropping of the right ventricular free wall of the transpericardial volume data in this case is visualized. An abnormal muscle bundle that separates the large VSD into the right and left halves exists from the center of the VSD to the right ventricular free wall. It was diagnosed as the subpulmonary VSD and the subaortic VSD, so-called multiple VSDs. Figure 1.6 is the view of the VSD from the pulmonary artery side, which is the surgeon’s view of the opened pulmonary artery. The VSD under the aortic valve is invisible due to the abnormal muscle bundle. The surgical findings shown in Fig. 1.7 are completely consistent with the preoperative echo findings when the pulmonary artery is opened. The VSD that is inserted with forceps was the one under the pulmonary valve, while the other one is not accessible being blocked by the muscle bundle as expected. The VSD under the aortic valve is approached from the tricuspid valve side and a route is created from the left to the right ventricle. On the other hand, patch closure is performed for the VSD under the pulmonary valve from the pulmonary valve side. If the two VSDs have not been found at the preoperative diagnosis, they could not have been closed completely and the patient could not have been disconnected from the cardiopulmonary bypass. Thus, when the VSD is multiple or the shapes of the defect and the surrounded structures are complicated, anatomically detailed diagnosis by optimal cropping of the transpericardial 3D volume data would be very useful for the surgery practically.