Fig. 4.1
Coarctation of the aorta in a neonate is shown by 3D volume-rendered (VR) MD-CT images. (a) VR image shows coarctation of the aorta and a long, hypoplastic arch between the left common carotid artery and left subclavian artery. (b) VR image shows severe coarctation of the aorta and hypoplastic distal arch and isthmus. Note that aortic arch anatomy is quite different among patients
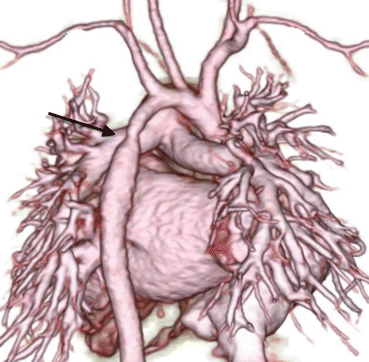
Fig. 4.2
A 1-month-old infant with ventricular septal defect presented with tachypnea and failure to thrive. The 3D volume-rendered image reveals coarctation of aorta (arrow) and mildly hypoplastic distal arch
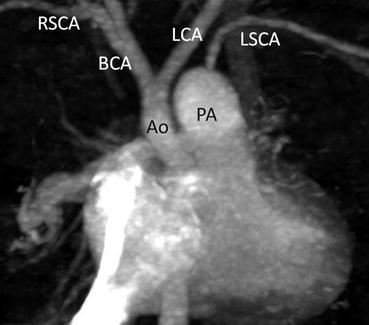
Fig. 4.3
A neonate with interruption of the aortic arch and ventricular septal defect. Coronal MPR images clearly show interruption of aortic arch between the left common carotid artery (LCA) and left subclavian artery (LSCA). Ao ascending aorta, BCA brachiocephalic artery, PA main pulmonary artery
Double aortic arch is one of the vascular rings that encircle the trachea and esophagus. It causes extrinsic airway obstruction (Figs. 4.4 and 4.5) that can be noninvasively and accurately diagnosed with MD-CT [12]. It is important for surgeons to know which arch is dominant, because thoracotomy is usually performed on the side of the more hypoplastic arch. An aberrant origin of the left subclavian artery associated with the right aortic arch can be missed on echocardiography; however, it can be easily diagnosed with MD-CT (Fig. 4.6), especially with 3D reconstructed images.
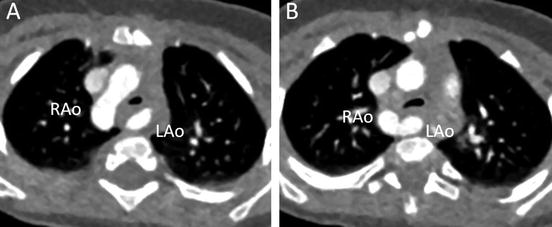
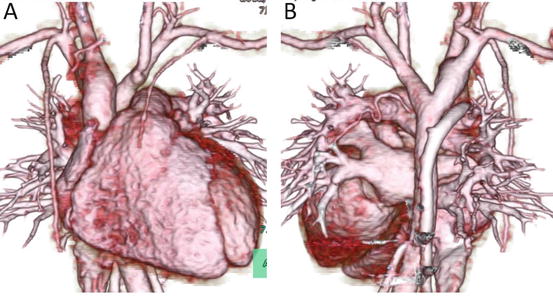
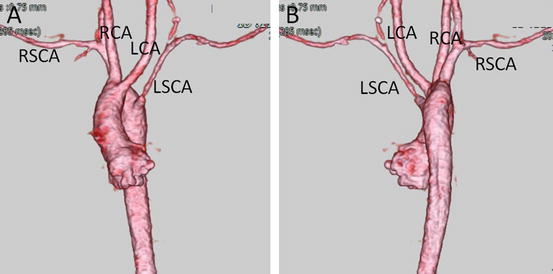

Fig. 4.4
A 7-month-old infant with stridor. Transaxial MD-CT images show the dominant right aortic arch (RAo) and smaller left aortic arch (LAo), with moderate tracheal narrowing

Fig. 4.5
3D volume-rendered images of the same patient as in Fig. 4.4, viewed from the anterior (a) and the posterior (b) perspectives. Note that the right aortic arch is larger than the left, which is not fully opacified because of an atretic portion

Fig. 4.6
Right aortic arch with aberrant origin of the left subclavian artery. 3D volume-rendered images show the left subclavian artery (LSCA) originating from the most dorsal part of the aortic arch, viewed from the anterior (a) and the posterior (b) perspectives. LCA left common carotid artery, RCA right common carotid artery, RSCA right subclavian artery
4.2.2 Pulmonary Artery
The pulmonary circulation in patients with pulmonary atresia is dependent on a patent ductus arteriosus (Fig. 4.7). Therefore, the Blalock-Taussig (BT) shunt operation is necessary during the neonatal period to maintain pulmonary blood flow. For preoperative evaluation, precise characterization of the pulmonary artery, including presence of juxtaductal pulmonary artery coarctation, is necessary. If juxtaductal pulmonary artery coarctation is present or likely to develop after BT shunt, plasty of the pulmonary artery should be performed at the same time as BT shunt with the patient on cardiopulmonary bypass [13, 14]. In contrast, if juxtaductal pulmonary artery coarctation is not present, only the BT shunt operation is performed, and cardiopulmonary bypass is no longer necessary. Evaluation by MD-CT allows selection of the optimal surgical strategy. Some patients with pulmonary atresia have nonconfluent pulmonary arteries (Fig. 4.8). MD-CT, especially 3D reconstruction, can provide information about the distance separating the two pulmonary arteries and their spatial relation with the surrounding structures. Thus, MD-CT makes it easier for surgeons to develop a preoperative strategy for pulmonary artery plasty.
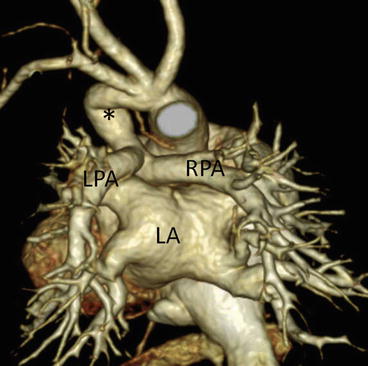
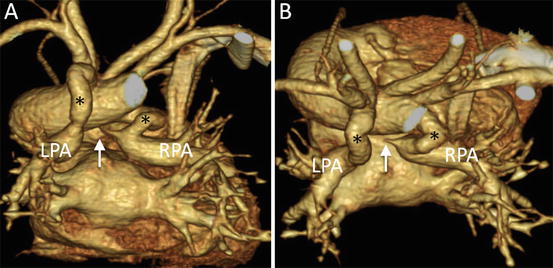

Fig. 4.7
A neonate with pulmonary atresia and ventricular septal defect. 3D volume-rendered image clearly shows patent ductus arteriosus (asterisk) and pulmonary arteries. No juxtaductal pulmonary artery coarctation is demonstrated. LA left atrium, LPA left pulmonary artery, RPA right pulmonary artery

Fig. 4.8
A neonate with pulmonary atresia, nonconfluent pulmonary artery, and univentricular heart. 3D volume-rendered images show discontinuity (arrow) of bilateral pulmonary arteries and tortuous bilateral patent ductus arteriosus (asterisk), viewed from the posterior (a) and superior (b) perspectives. LPA left pulmonary artery, RPA right pulmonary artery
MD-CT is useful for noninvasive assessment of pulmonary arterial stenosis and growth after corrective surgery or BT shunt (Figs. 4.9 and 4.10). It can indicate the exact site length and severity of stenosis. Echocardiography does not always provide sufficient images because of a limited echo window. Therefore, precise characterization of target lesions by MD-CT allows optimal preparation for surgery or catheter intervention.
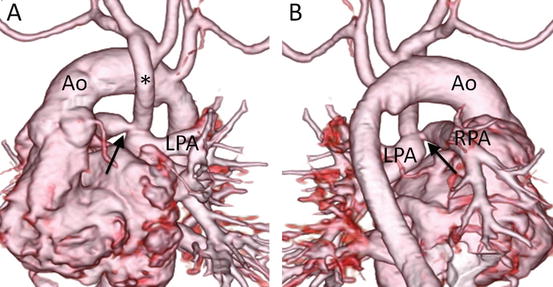
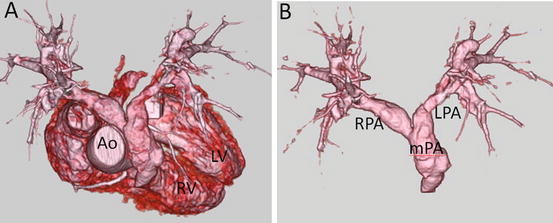

Fig. 4.9
A 1-month-old infant with pulmonary atresia and univentricular heart who underwent Blalock-Taussig shunt. 3D volume-rendered images, viewed from the anterior (a) and posterior (b) perspectives, clearly show discrete stenosis of the right pulmonary artery (RPA)

Fig. 4.10
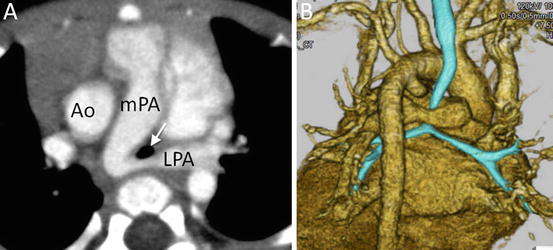
A 12-year-old boy with tetralogy of Fallot who underwent intracardiac repair. 3D volume-rendered images show bilateral pulmonary stenosis. Ao ascending aorta, LPA left pulmonary artery, LV left ventricle, mPA main pulmonary artery, RPA right pulmonary artery
4.2.3 Pulmonary Vein
MD-CT is a very useful diagnostic tool in total anomalous pulmonary venous connection (TAPVC). Although TAPVC can only be diagnosed by echocardiography, it may be difficult to determine the precise structures of pulmonary veins, especially in mixed type (Fig. 4.11) or when TAPVC is associated with heterotaxy syndrome (Figs. 4.12 and 4.13). In such cases, MD-CT 2D images as well as 3D reconstruction can provide accurate anatomical information [2, 15, 16]. It is an excellent diagnostic tool for preoperative evaluation and for guidance of the surgical operation, because the drainage site of the pulmonary vein can be exactly determined. Furthermore, MD-CT can provide interventionalists with important information. In a neonate with TAPVC, stent implantation may be one of the treatment options to relieve stenosis, which is often found at the drainage site of pulmonary vertical veins [17]. Interventionalists must pay attention not only to the stenotic site but also to the surrounding structures (e.g., bronchi, coronary arteries, branches of the pulmonary vein, systemic veins, etc.) [18–20]. After repair of TAPVC, pulmonary vein stenosis may develop in some patients (Fig. 4.14), and MD-CT can give useful information about the site and severity of stenosis.
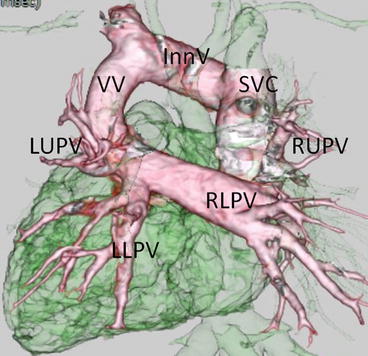
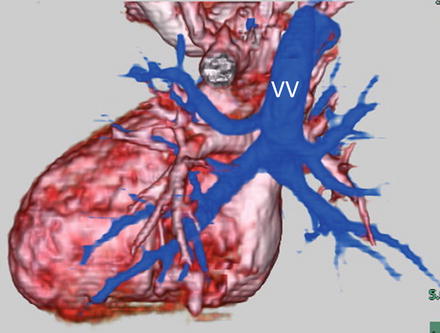
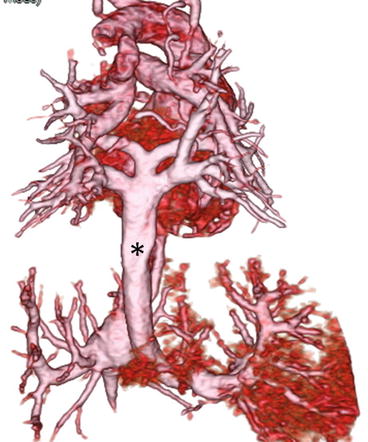
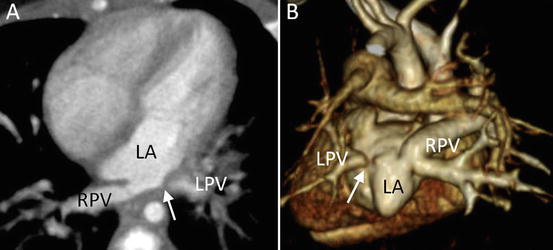

Fig. 4.11
A neonate with mixed type total anomalous pulmonary venous connection. 3D volume-rendered images show the right lower pulmonary vein (RLPV) and left upper (LUPV); the left lower pulmonary veins (LLPV) connect to a horizontal confluence, which leads into a vertical vein (VV). The VV drains into the innominate vein (InnV). The right upper pulmonary vein (RUPV) drains into the superior vena cava (SVC)

Fig. 4.12
A neonate with supracardiac total anomalous pulmonary venous connection. 3D volume-rendered MD-CT images viewed from the posterior perspective show all four pulmonary veins connect to a vertical vein (VV), which drains into the superior vena cava

Fig. 4.13
A neonate with infracardiac total anomalous pulmonary venous connection. 3D volume-rendered MD-CT images viewed from the posterior perspective show all four pulmonary veins connected to a vertical confluence, which leads into a vertical vein (asterisk). The vertical vein descends and drains into the portal vein

Fig. 4.14
A 2-month-old infant with total anomalous pulmonary venous connection, who developed pulmonary venous obstruction after surgical repair. (a) Transaxial MD-CT image shows severe stenosis of the left pulmonary vein (arrow). (b) 3D volume-rendered MD-CT images (from behind) demonstrate the obstructed left pulmonary vein (arrow). LA left atrium, LPV left pulmonary vein, RPV right pulmonary vein
4.2.4 Coronary Arteries
MD-CT can provide us with precise images concerning the origin of coronary arteries. In patients with anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA), the diagnosis can often be made only by echocardiography; however, this is not always the case. The left coronary artery rarely originates from the right pulmonary artery in ALCAPA (Fig. 4.15) [21]. Such cases may be difficult to diagnose by echocardiography only, because the left coronary artery looks as if it originates normally from the left coronary cusp on echocardiography. MD-CT is a very useful tool for the diagnosis of such cases.
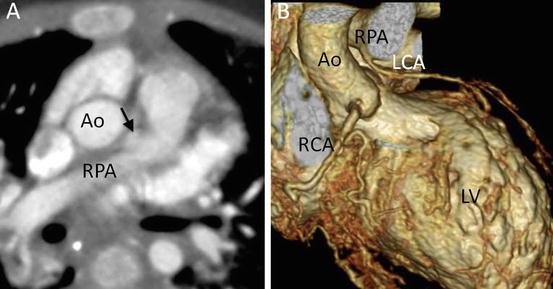

Fig. 4.15
A 2-month-old infant presented with severe congestive heart failure. (a) Transaxial MD-CT image shows an anomalous origin of the left coronary artery (arrow) from the right pulmonary artery (RPA). (b) 3D volume-rendered MD-CT image clearly shows an anomalous origin of the LCA from the RPA
In the field of pediatric cardiology, echocardiography is a noninvasive and very useful tool to visualize the coronary arteries. Echocardiography is useful in the diagnosis of enlarged lesions; however, there are some limitations, such as the diagnosis of stenotic lesions in the coronary arteries. Furthermore, it is more difficult to obtain clear images in adolescents or patients with chest deformities such as pigeon chest. However, MD-CT can depict small structures such as the coronary arteries with improved spatial resolution because of increased numbers of detector rows [22–25]. Multiplanar reformatted (MPR), maximum-intensity projection (MIP), and 3D volume-rendered (VR) images are powerful tools that provide additional information on the nature and extent of disease and accurately illustrate anatomical relationships (Figs. 4.16, 4.17, 4.18, 4.19, and 4.20).
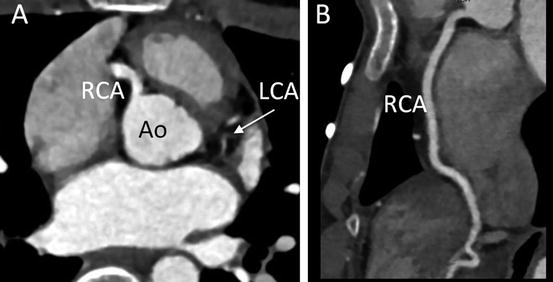
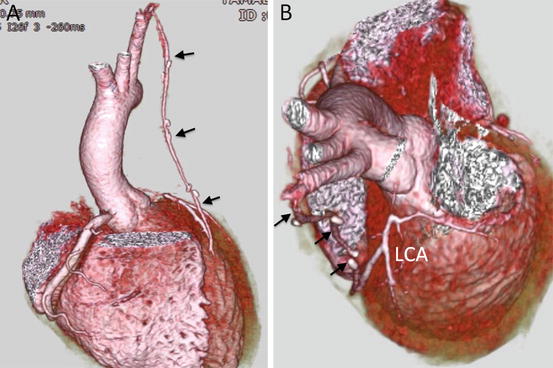
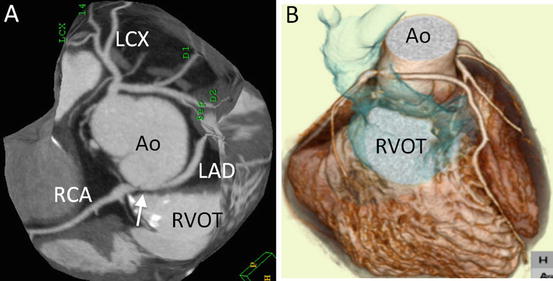
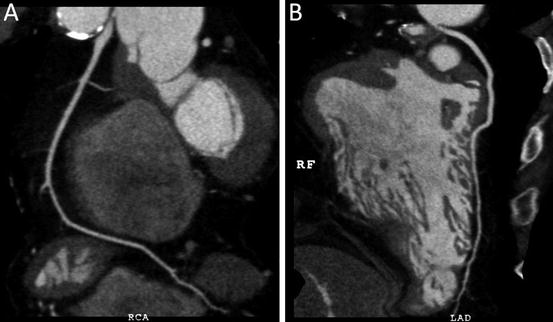
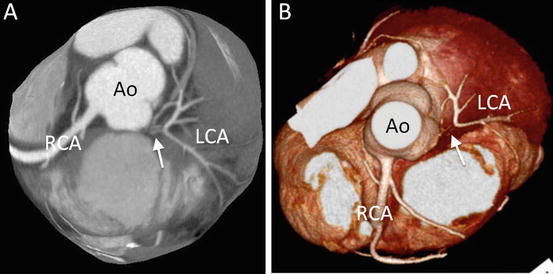

Fig. 4.16
A 14-year-old boy presented with chest pain on exercise. (a) Transaxial MD-CT image demonstrates discontinuity between the ascending aorta (Ao) and left coronary artery (LCA), which is hypoplastic. He was diagnosed with congenital ostial atresia of the LCA. (b) Curved multiplanar reconstruction image shows an intact right coronary artery

Fig. 4.17
3D volume-rendered image shows the status of the patient in Fig. 4.16 after a left internal mammary artery bypass graft (arrow) to the diagonal coronary artery

Fig. 4.18
A 28-year-old man with tetralogy of Fallot and pulmonary atresia who underwent Rastelli operation. (a) Slab maximum-intensity projection (MIP) image shows stenosis at the origin of right coronary artery and left anterior-descending coronary artery (LAD), which anomalously originates from the right coronary cusp and has an interarterial course between the aorta (Ao) and Rastelli conduit (in blue). (b) 3D volume-rendered image also shows the interarterial course of the LAD

Fig. 4.19
Curved multiplanar reconstruction images (the same patient as in Fig. 4.18) show the entire course of the right coronary artery (in (a)) and left anterior-descending coronary artery (in (b)). Note that only the origins of both coronary arteries are stenotic

Fig. 4.20
An 18-year-old man with transposition of the great arteries who underwent arterial switch operation. (a) Transaxial multiplanar reformatted CT image shows obstruction of main trunk (arrow) of the left coronary artery (LCA). (b) 3D volume-rendered CT image (from above). Ao ascending aorta, RCA right coronary artery
4.2.5 Systemic Venous Return
Anomalies of systemic venous return may be associated with congenital heart disease [26]. The general population without congenital heart disease may have a left superior caval vein (LSVC); however, it is more prevalent in individuals with congenital heart disease. The LSVC usually drains into the coronary sinus; however, it may drain into the left atrium or atrial roof (Fig. 4.21). It is very important to evaluate its drainage site and presence of the innominate vein for intracardiac repair. When the LSVC drains into the left atrium, atrial septation results in desaturation. MD-CT can illuminate the precise site of LSVC drainage.
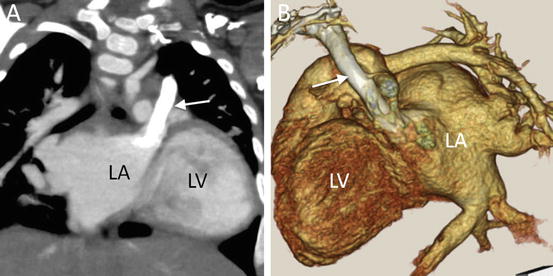

Fig. 4.21
A 9-month-old infant with partial atrioventricular septal defect. (a) Coronal multiplanar reformatted MD-CT image shows the left superior vena cava (arrow) draining not into coronary sinus but into the roof of left atrium (LA). (b) Oblique sagittal 3D volume-rendered CT image. LV left ventricle
4.2.6 Airways (Spatial Relationship with the Great Arteries)
In patients with congenital heart disease, anomalous vessels and previous surgical operations may cause airway obstruction [3, 6, 27, 28]. MD-CT can provide information on not only abnormal arteries or veins but also the structures of the trachea, bronchi, and coronary arteries. Pulmonary artery sling (anomalous origin of the left pulmonary artery from the right pulmonary artery) causes compression of the trachea by the anomalous origin of the left pulmonary artery (Fig. 4.22) [3, 6, 28]. 3D images superimposed on the obstructed airway show the spatial relationships. In patients with tetralogy of Fallot and absent pulmonary valve, the airway is compressed and obstructed by the dilated main and peripheral pulmonary arteries (Fig. 4.23) [3]. The site of airway obstruction can be clearly visualized with MD-CT, especially 3D images superimposed on images of the airway (Fig. 4.24). In patients with double aortic arch, the airways are surrounded and compressed by both aortic arches (Fig. 4.25).