Chapter 5 Radiofrequency Thermal Ablation
Historical Background
Investigators in the 1960s and 1970s observed third-degree skin burns and saphenous nerve injuries after thermal ablation of the saphenous vein.1,2 Low-wattage, bipolar current, and specific electrode designs, coupled with algorithms governed by frequent sampling of wall temperature and impedance, were expected to mitigate thermal damage to adjacent tissues. The use of bipolar electrodes, which concentrate current density along minimal impedance paths between the poles, helped resolve these problems.3 In addition, early experiences demonstrated that procedural modifications were also needed to minimize complications and early failures.
First-Generation Device
In an industry-sponsored feasibility study: (1) the Restore catheter (VNUS Medical Technologies, Inc., Sunnyvale, CA) induced a short subvalvular constriction to improve the competence of valve leaflets and (2) the Closure catheter applied resistive heating over long vein lengths to cause maximum wall contraction for permanent obliteration.4 Treatment with Restore catheters resulted in recurrent or persistent reflux in 81% of patients followed up for 6 to 12 months. Treatment with Closure catheters resulted in a 6% recurrent reflux rate and a 4% incidence of recurrent varicosities at a mean follow-up of 4.7 months. The authors concluded that treatment with Closure catheters was an effective, less invasive option than saphenous stripping, with complications and early failures that could be mitigated through further procedural modifications.
In 1999, VNUS Medical Technologies, Inc. (Sunnyvale, CA) received approval from the U.S. Food and Drug Administration (FDA) to market and sell a new device for closure of incompetent saphenous veins. The first-generation device, known as the Closure Procedure, used bipolar electrodes mounted on the end of a catheter to deliver radiofrequency (RF) energy to the inner vein wall. The catheter’s collapsible bipolar electrodes included a temperature sensor that provided feedback to a dedicated RF generator. When deployed, the electrodes made direct contact with the endoluminal surface of the vein wall, and RF energy was delivered. The resistive effects of the vein wall tissue caused conversion of RF energy into heat. The principal mechanism of RF ablation has been demonstrated in animal studies.5 Vein wall collagen contraction in response to thermal energy causes immediate vein wall thickening and reduction in the lumen diameter. Endothelial destruction causes an inflammatory response, which results in fibrosis and permanent vein occlusion.
The thermal effect on the vein wall is directly related to both the treatment temperature and the treatment time, the latter being a function of catheter pullback speed. The treatment protocol called for a treatment temperature of 85 °C at a pullback speed of 3 cm/min. The thermal effect produces sufficient collagen contraction to occlude the lumen while limiting heat penetration to perivenous tissue.6,7 To assess the potential of perivenous tissue damage, the adventitial temperature was recorded in an in vitro model.8 With the standard treatment protocol, the average peak adventitial temperature was 64.4° C and usually lasted for approximately 10 seconds at any given position along the length of the vein. Peak adventitial temperature was decreased to 51.3° C in the presence of a 2-mm perivenous saline layer.
Second-Generation Device (VNUS ClosureFAST)
The linear endovenous energy density (LEED) is frequently used to compare energy dosing in endovenous procedures. With the first-generation (bipolar) RF device, the catheter pullback velocity had to be slow enough to allow resistive heating of the vein wall to a target temperature of 85 °C. Measurements of the delivered energy dose to the vein were not displayed to the operator because the power delivered by the generator was subject to regulation by a feedback loop to maintain a constant temperature of 85° C. With CLF, the temperature is kept stable at 120° C during a 20-second treatment cycle. At the saphenofemoral junction (SFJ), two cycles of RF energy are delivered, averaging an LEED of 116.2 ± 11.6 J/cm for the first 7 cm of vein juxtaposed to the SFJ to ensure good vein closure at this critical site.9 Distal to the SFJ, 68.2 ± 17.5 J/cm is delivered to each 7-cm treatment site. Thus, this aggressive “double energy cycle” at the zone of the SFJ is supported by Almeida and Raines’ retrospective analysis.10
Proebstle reported outcomes following CLF in early 2008. The occlusion rate following segmental RFA was 99.6% at 2 years, and 70% of treated patients did not require any analgesia postprocedure.9
Quality-of-Life Changes
Studies of quality of life are scarce, but significant improvements in disease-specific quality-of-life following RFA were reported in the EVOLVeS study, using the CIVIQ-2 questionnaire.11,12 Moreover, these quality-of-life statistics were improved compared to patients treated with traditional venous surgery.
Etiology and Natural History of Disease
Treatment Efficacy
Duplex ultrasound examination has significantly advanced our understanding of venous disease and provides both anatomic and pathophysiologic information. The morphologic and hemodynamic outcomes following RF ablation have been described in detailed ultrasound studies by Pichot et al.13 The pathologic sequelae of a treated vein are reflected by its sonographic progression. Occluded veins were initially hypoechogenic compared with the surrounding tissue and gradually evolved into hyperechogenic and eventually isoechogenic presentations, indicating a healing process. Approximately 60% of veins were hypoechogenic and 40% were hyperechogenic at 1 week. By 6 months, they became either hyperechogenic or isoechogenic.13 Sonographic disappearance of the saphenous vein, the desired endpoint, was observed by Weiss and Weiss in 90% of limbs at 2 years.14
Failures
Incomplete ablation, either segmental or total length of the vein, constitutes anatomic failure. Veins that are patent after treatment represent initial incomplete treatment or subsequent recanalization. Regardless of the presence of an anatomic failure, clinically, symptom improvement was often demonstrated in patients reported in the registry.15
Four types of SFJ morphologies were identified after RF ablation15–17: J-1, defined as complete SFJ obliteration with no SFJ flow; J-2, defined as patent SFJ tributaries draining toward the femoral vein with (J-2b) or without (J-2a) a short patent saphenous stump; and J-3, defined as terminal great saphenous vein (GSV) competence with normal antegrade flow coming from both tributaries and the saphenous vein above a limited GSV obliteration. Two years after RF ablation, the most common findings were either complete SFJ obliteration or a 5-cm or smaller patent terminal stump connecting prograde tributary flow through the SFJ, accounting for approximately 90% of the limbs treated.15,17
The clinical significance of a short patent SFJ stump was analyzed in a study by Merchant et al.15 A total of 319 limbs in the Clinical Registry were followed at 1 week, 6 months, 1 year, and 2 years, with 2-year data available for 121 limbs. Comparison of symptom improvement and varicose vein absence demonstrated no statistically significant differences between patients with complete SFJ obliteration and those with a short patent SFJ stump at any follow-up time point.
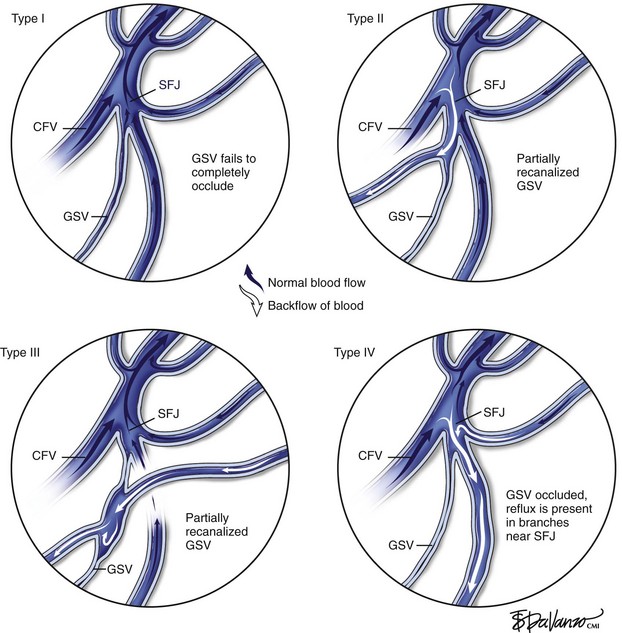
Merchant and Pichot18 categorized anatomic failure after an RF ablation procedure into three types. Type I failure (nonocclusion) refers to a vein that fails to occlude because of suboptimal technique, such as a rapid pullback speed resulting in an insufficient delivery of thermal dose. It has also been observed that in a very small percentage of patients, veins may be nonresponsive to thermal ablation; it has been postulated that the collagen structure might be different in these patients. Of veins that recanalized (Type II failure), 23% were associated with either tributary or perforator incompetence, accounting for 70% of the total anatomic failures.
On the other hand, Type II and Type III failures were risk factors for varicose vein recurrence. Type II failure patients were 3.8 times and Type III failures were 4.0 times as likely to develop varicose vein recurrence compared to patients with anatomic success. Type I failure did not reach statistical significance in this analysis. One possible explanation is lack of follow-up. Most patients had follow-up of less than 3 years. Further, some patients may have been treated with other methods and lost to follow-up. In this setting, the impact of early failure on varicose vein recurrence may not have been identified. Surveillance monitoring, early recognition of anatomic failure, and taking further corrective action that may include RF ablation retreatment may prevent or reduce varicose vein recurrence. However, it should be recognized that disease progression is likely to play a major role in Type III failure and may also account for some of Type II failure. This may contribute to an increase in varicose vein recurrence at 4 and 5 years (Fig. 5-1).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree