Using radiofrequency–intravascular ultrasound (VH-IVUS), we have previously demonstrated that in 50% of patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention with optimal angiographic result, the stent does not fully cover the whole VH-IVUS-derived thin-cap fibroatheroma (VH-TCFA) related to the culprit lesion. Presently, we set out to extend these findings to 20 patients with non-STEMI with Thrombolysis In Myocardial Infarction flow 3 in the infarct-related artery before intervention who were then treated with angiography-guided direct stent implantation. The lesion was imaged with VH-IVUS before and after intervention, but the results were blinded to the operator. Plaque rupture site was identified in 8 lesions (40%), all proximal to the minimum lumen area (MLA) site. The maximum necrotic core site was found proximal to MLA in 18 lesions and at the MLA in 2 lesions. Although the plaque rupture site was fully covered with the stent in all lesions, an uncovered VH-TCFA was found in 7 lesions (35%), 4 in the proximal reference segment, 1 in the distal reference segment, and 2 in both the proximal and distal reference segments. In conclusion, in 35% of patients with non-STEMI undergoing angiography-guided emergent percutaneous coronary intervention, the stent does not fully cover a VH-TCFA related to the culprit lesion.
Most acute coronary artery occlusions occur because of a rupture of an atherosclerotic plaque followed by thrombus formation. Postmortem studies have shown that the occlusion is mainly composed of a thrombus, whereas the plaque rupture is located proximal or distal to the occlusion and is not necessarily lumen compromising. Therefore, when treating the culprit lesion with stent implantation, incomplete stent coverage of the true culprit lesion (culprit of the culprit or the site of plaque rupture) may occur when only the occlusion or the minimum lumen area (MLA) site is treated under angiographic guidance. This could be one of the possible mechanisms responsible for future cardiac events. We have previously demonstrated using radiofrequency–intravascular ultrasound (VH-IVUS) that in 50% of patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI) with optimal angiographic result, the stent does not fully cover the whole thin-cap fibroatheroma (TCFA) related to the culprit lesion. Currently, we extend this investigation to patients with non-STEMI (NSTEMI). The purpose of the present study was to use intravascular ultrasound (IVUS) and VH-IVUS to assess culprit lesion coverage after angiography-guided emergent PCI in patients with NSTEMI.
Methods
The present study was a single-center, prospective, observational registry. The study protocol was approved by the Institutional Review Board of the Jagiellonian University Medical College in Krakow (KBET/63/B/2008) and conformed to the statute of the Declaration of Helsinki. All patients provided written informed consent before enrollment.
Patients aged >18 years with uncomplicated NSTEMI within 72 hours after onset of symptoms qualifying for emergent PCI were eligible. Patients were not eligible if angiography demonstrated left main coronary artery stenosis of >50%, Thrombolysis In Myocardial Infarction (TIMI) flow <3 in the infarct-related artery, or if the coronary anatomy of the culprit vessel was inappropriate for IVUS assessment or stent implantation.
Culprit lesions were de novo, nonostial, and without heavy calcification located in the proximal or midsegment of the patent infarct-related artery with a reference vessel diameter of ≥2.5 mm by visual estimation. IVUS pullback was conducted before stent implantation. Stent length and diameter selection was based on angiography alone and was followed by direct stent implantation with postdilation as needed to achieve optimal angiographic result (residual angiographic diameter stenosis of <20% and TIMI flow 3). After finishing the procedure, IVUS pullback was repeated. Operators performing emergent PCI were blinded to IVUS and VH-IVUS findings. Therefore, these findings did not impact PCI, which was carried out according to the standard practice of the center.
IVUS was conducted using the commercially available Eagle Eye Gold phased array IVUS probe (Volcano Corp., Rancho Cordova, California). The probe was advanced >10 mm distal to the distal end of angiographic culprit lesion and motorized pullback up to the guiding catheter was performed at the speed of 0.5 mm/s. Radiofrequency backscatter data were collected simultaneously and triggered by the R-wave peak of the patient’s electrocardiogram using a dedicated IVUS console (Rancho Cordova, California). The region of interest was defined in each vessel as the stented lesion plus 10 mm proximal and distal to the edges of the stent. Each region of interest imaged by IVUS and VH-IVUS was analyzed by 2 different analysts to address inter- and intraobserver variabilities. Planar and volumetric IVUS analyses were performed according to the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). Planar and volumetric VH-IVUS analyses were performed according to the Tissue characterization using intravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting document . The IVUS analysis was performed using echoPlaque 4 software (INDEC Medical Systems, Santa Clara, California). The VH-IVUS analysis was performed using pcVH 2.2 and qVH softwares (Rancho Cordova, California) for tissue characterization and advanced analysis, respectively. IVUS and VH-IVUS data were analyzed by 2 independent analysts (JJ and MW) blinded to the clinical data and procedural information, and all analyses were reviewed by a single independent reviewer. Overall, inter- and intraobserver variabilities showed good intraclass correlation among analysts and for the same analyst at the core laboratory with higher level of agreement obtained for TCFA detection for intraobserver analysis and lower for interobserver analysis (κ = 0.933 and 0.894, respectively).
Off-line qualitative and quantitative coronary angiographic (QCA) analysis was performed according to the well-established protocol. QCA analysis was performed using the Sanders Data Systems QCAPlus software (Palo Alto, California) by an experienced analyst (LR) blinded to clinical data and procedural information.
Statistical analysis was performed using the JMP software, version 9.0.0 (SAS Institute, Cary, North Carolina). Data obtained from the study were analyzed using standard methods of descriptive statistics. Categorical variables were compared using the chi-square or Fisher’s exact test. These data are presented as percentages. Continuous variables are presented as mean ± 1 SD. These variables were analyzed with 2-sided Student t or Mann-Whitney test. Compliance with the normal distribution was confirmed using Kolmogorov-Smirnov test.
Results
Of 32 patients with NSTEMI who were screened, 20 were included in the study in compliance with all inclusion and exclusion criteria. Main reasons for screening failures were vessel tortuosity, excessive calcifications, and subtotal vessel stenosis that did not allow crossing with an IVUS probe. Procedural data are listed in Table 1 . Final TIMI flow 3 was achieved in all lesions. No death, reinfarction, or repeat interventions were reported during in-hospital, at 30-day, or at 1-year follow-up.
| Age (Yrs) | Gender | IRA | Thrombectomy | Stent |
|---|---|---|---|---|
| 41 | M | LC | No | BMS |
| 41 | M | LC | Yes | BMS |
| 42 | M | LAD | No | DES |
| 47 | M | LC | No | BMS |
| 48 | M | LC | No | BMS |
| 54 | M | LC | No | BMS |
| 55 | W | LAD | No | DES |
| 57 | M | LAD | No | BMS |
| 57 | M | LC | No | BMS |
| 58 | M | LAD | No | DES |
| 59 | M | LC | No | BMS |
| 61 | M | LAD | No | BMS |
| 64 | M | LC | No | BMS |
| 64 | M | LAD | No | DES |
| 67 | W | Right CA | Yes | DES |
| 69 | M | LAD | No | BMS |
| 70 | W | Right CA | No | BMS |
| 71 | M | LAD | No | DES |
| 75 | W | LC | No | BMS |
| 77 | M | LC | No | BMS |
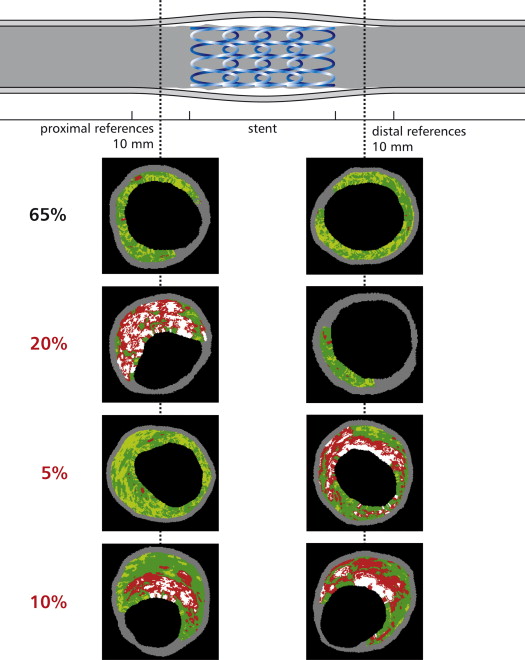
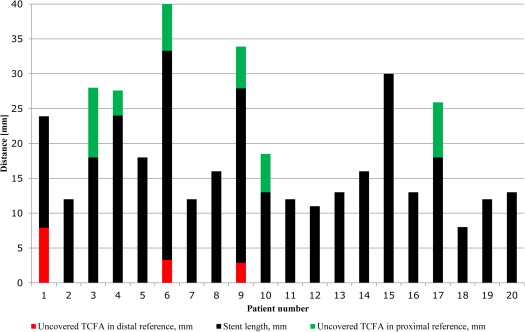
After angiography-guided stent implantation, blinded IVUS imaging showed that the plaque rupture site was identified in 8 lesions (40%), all proximal to the MLA site, and in all cases was fully covered with the stent. However, an uncovered VH-IVUS-derived TCFA (VH-TCFA) was found within 10 mm proximal to the stent in 4 lesions (20%), within 10 mm distal to the stent in 1 lesion (5%), and both proximal and distal to the stent in 2 lesions (10%; Figure 1 ). The mean length of the segment with an uncovered VH-TCFA was 6.1 ± 2.5 mm (maximum 12.1 mm). Figure 2 shows the lengths of the stents and the lengths of the segments with an uncovered VH-TCFA proximal and/or distal to the stent for individual patients. The maximum necrotic core site was proximal to the MLA site in 18 lesions (90%), at the MLA site in 2 lesions (10%), and distal to the MLA site in no lesion. Thus, the plaque rupture and maximum necrotic sites were mainly located proximal to the site of MLA (on average by 3.70 ± 2.90 and 4.68 ± 3.10 mm, respectively). The distribution of distances from the plaque rupture site and the maximum necrotic core site to the MLA site for individual patients is shown in Figure 3 . The distance between the MLA site and the plaque rupture sites was smaller than that between the MLA and maximum necrotic core sites.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


