56 Qualitative and Quantitative Coronary Angiography
 Qualitative Angiography
Qualitative Angiography
Update On The Acc/Aha Task Force On Lesion Morphology
A joint task force of the American College of Cardiology (ACC) and American Heart Association (AHA) established criteria to estimate procedural success and complication rates after balloon angioplasty based on the presence or absence of specific high-risk lesion characteristics.1 Although these criteria were developed based solely on the task force’s clinical impressions (Table 56-1), their estimates of procedural success and complications were closely correlated with the procedural outcomes demonstrated in patients undergoing multivessel balloon angioplasty.2 Chronic total occlusion, high-grade stenosis, stenosis on a bend of 60 degrees or more, and location in vessels with proximal tortuosity were associated with an adverse outcome.2 The most complex lesion morphologies (i.e., “type C” lesions) were associated with less satisfactory procedural outcomes.3,4 Definitions for these variables are provided (Table 56-2). With improved outcomes associated with the use of coronary stents, contemporary composite risk scores were proposed.5,6 The Society for Cardiac Angiography and Interventions (SCAI) registry evaluated 61,926 patients (74.5% received stents) from the ACC National Cardiovascular Data Registry and classified their lesions into four groups: non-type C patent, type C patent, non-type C occluded, and type C occluded (Table 56-3).5 These more simplified criteria provided better discrimination for success or complications than the ACC/AHA original classification, with a c statistic of 0.69 for success using the ACC/AHA original classification system, 0.71 using the modified ACC/AHA system, and 0.75 for the SCAI classification.5 The Mayo Clinic risk score was constructed by adding integer scores for the presence of eight morphological variables and was compared with the ACC/AHA risk score in 5,064 patients undergoing PCI, of whom 183 (4%) experienced an adverse event (death, Q-wave myocardial infarction, stroke, emergency CABG).6 The Mayo Clinic risk score offered significantly better risk stratification than the ACC/AHA lesion classification for the development of cardiovascular complications, whereas the ACC/AHA lesion classification was a better system for determining angiographic success.6
TABLE 56-1 ACC/AHA Characteristics of Type A, B, and C Coronary Lesions
| Type A lesions—high success (≈85%), low risk | |
| Discrete (<10 mm) | Little or no calcium |
| Concentric | Less than totally occlusive |
| Readily accessible | Not ostial in location |
| Nonangulated segment (<45 degrees) | Smooth contour, no major side branch |
| Absence of thrombus | No side branch involvement |
| Type B lesions—moderate success (60%–85%), moderate risk | |
| Tubular (10- to 20-mm length) | Moderate to heavy calcification |
| Eccentric | Total occlusion <3 months old |
| Moderate tortuosity of proximal segment | Moderately angulated (45–90 degrees) |
| Bifurcation lesion requiring double guidewire | Irregular contour |
| Some thrombus present | |
| Type C low success (≈60%), high risk | |
| Diffuse (>20-mm length) | Total occlusion >3 months old |
| Excessive tortuosity of proximal segment | Inability to protect major side branches |
| Extremely angulated segment (>90 degrees) | Degenerated vein grafts with friable lesions |
Modified from Ryan TJ, Faxon DP, Gunnar RP, et al. Guidelines for percutaneous transluminal coronary angioplasty: a report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). J Am Coll Cardiol. 1988;12:529–545.
TABLE 56-2 Definitions of Preprocedural Lesion Morphology
| Variable | Definition |
|---|---|
| Eccentricity | Stenosis that is noted to have one of its luminal edges in the outer quarter of the apparently normal lumen |
| Irregularity | Characterized by lesion ulceration, intimal flap, aneurysm, or “sawtooth” pattern |
| Ulceration | Lesion with a small crater consisting of a discrete luminal widening in the area of the stenosis provided that it does not extend beyond the normal arterial lumen |
| Aneurysmal dilation | Segment of arterial dilation larger than the dimensions of the normal arterial segment |
| Sawtooth pattern | Multiple sequential stenotic irregularities |
| Lesion length | Measured “shoulder-to-shoulder” in an unforeshortened view |
| Discrete | Lesion length <10 mm |
| Tubular | Lesion length 10–20 mm |
| Discrete | Lesion length >20 mm |
| Ostial location | Origin of the lesion within 3 mm of the vessel origin |
| Lesion angulation | Vessel angle formed by the center line through the lumen proximal to the stenosis and extending beyond it and a second center line in the straight portion of the artery distal to the stenosis |
| Moderate | Lesion angulation >45 to 90 degrees |
| Severe | Lesion angulation >90 degrees |
| Bifurcation | Present if a medium or large branch (>1.5 mm) originates within the stenosis and if the side branch is completely surrounded by stenotic portions of the lesion to be dilated |
| Lesion accessibility | |
| Moderate tortuosity | Lesion is distal to two bends <75 degrees |
| Severe tortuosity | Lesion is distal to three bends >75 degrees |
| Degenerated SVG | Graft characterized by luminal irregularities or ectasia comprising >50% of the graft length |
| Calcification | Readily apparent densities noted within the apparent vascular wall at the site of the stenosis |
| Moderate | Densities noted only with cardiac motion before contrast injection |
| Severe | Radiopacities noted without cardiac motion before contrast injection |
| Total occlusion | TIMI 0 or 1 flow |
| Thrombus | Discrete, intraluminal filling defect is noted with defined borders and is largely separated from the adjacent wall; contrast staining may or may not be present |
SVG, saphenous vein graft; TIMI, Thrombolysis in Myocardial Infarction.
TABLE 56-3 SCAI Lesion Classification System: Class I to IV Lesions
| Type I lesion (highest success expected, lowest risk) |
| Type II lesion |
| Type III lesion |
| Type IV lesion |
Modified from Krone R, Shaw R, Klein L, et al. Evaluation of the American College of Cardiology/American Heart Association and the Society for Coronary Angiography and Interventions lesion classification system in the current “stent era” of coronary interventions (from the ACC National Cardiovascular Data Registry). Am J Cardiol. 2003;92:389–394.
The Syntax Score
The SYNTAX score was developed to incorporated both the complexity and extent of coronary artery disease in patients undergoing PCI or CABG.7,8 A lesion is defined as significant when it causes a >50% reduction in luminal diameter by visual assessment in a vessel >1.5 mm in diameter.8 A multiplication factor of 2 is used for nonocclusive lesions and 5 for occlusive lesions reflecting the difficulty of the percutaneous treatment.8 Up to 12 lesions are identified within the coronary tree, and each is assessed for its severity, including the presence of a total occlusion (with appropriate characterization) and of side branches and their size.8 Each lesion is also weighted by its contribution to the myocardial bed that it supplies.8 In a right-dominant system, the right coronary artery (RCA) supplies approximately 16% and the left coronary artery (LCA) 84% of the flow to the left ventricle (LV), with 66% to the left anterior descending artery (LAD) and 33% to the left circumflex coronary artery (LCX). These factors are included in the weight given to each segment. Lesions are further graded by their complexity, including multiple tandem lesions, morphology of total occlusions, bifurcation and trifurcation involvement, aorto-ostial location, diffuse disease and small vessels, severe tortuosity, length >20 mm, heavy calcification, and thrombus.8,9 The predictive value of the SYNTAX score in patients who had undergone PCI for three-vessel disease was evaluated for 1,292 lesions in 306 patients in the Arterial Revascularization Therapies Study Part II.10 The rate of major adverse cardiac and cerebrovascular events at 370 days was 27.9% in the tertile with the highest SYNTAX score and 8.7% in the tertile with the lowest SYNTAX score (hazard ratio [HR] 3.5, P = 0.001).10 By multivariate analyses, SYNTAX score independently predicted outcome and the risk of major adverse cardiac and cerebrovascular events. Compared with the modified lesion classification scheme of the American Heart Association/American College of Cardiology, the SYNTAX score showed a greater discrimination ability and better goodness of fit.10 The SYNTAX score has been used to predict outcomes in patients enrolled in studies of drug-eluting stents.11,12 In an analysis of 819 patients with left main coronary artery disease who underwent revascularization in two Italian centers, the outcomes of patients undergoing PCI and CABG were studied. In patients with SYNTAX scores ≤ 34, the rates of 2-year mortality were similar between CABG and PCI (6.2% vs. 8.1%, respectively; P = 0.461).11 Among patients with SYNTAX scores >34, those treated with CABG had lower rates of mortality than those treated with PCI (8.5% vs. 32.7%, respectively; P < 0.001).11 A similar correlation with the SYNTAX score and surgical outcome was not found in patients undergoing CABG.13,14 The SYNTAX score has also been incorporated into the recommendations by the European Society of Cardiology guidelines15 and to evaluate patients as suitable candidates for CABG or PCI in clinical practice.16 An improvement in the ability of the SYNTAX score to predict major adverse cardiovascular and cerebrovascular events (MACCE) and mortality can be achieved by combining the SYNTAX score with a simple clinical risk score incorporating age, ejection fraction, and creatinine clearance to produce the clinical SYNTAX score (CSS).17 The CSS incorporates the SYNTAX score with a modified ACEF score ([age/ejection fraction] +1 for each 10 mL the creatinine clearance is <60 mL/min per 1.73 m2) to estimate major adverse cardiac events at 1 and 5 years after PCI.17 At 1-year follow-up, rates of repeat revascularization and MACCE were significantly higher in the highest-tertile group.17 At 5-year follow-up, those with high CSSs had a comparable rate of myocardial infarction, a trend toward a significantly higher rate of death, and significantly higher rates of repeat revascularization and overall MACCE compared with patients in the lower two tertiles.17 The respective c statistics for the CSS, SYNTAX score, and ACEF score for 5-year mortality were 0.69, 0.62, and 0.65; for 5-year MACCE, they were 0.62, 0.59, and 0.57.
Risk Assessment Using Specific Lesion Morphological Criteria
Despite the value of risk scores in estimating aggregate procedural risk, there are several limitations of these criteria as applied to individual patients. Identification of lesion characteristics—such as eccentricity, irregularity, angulation, and tortuosity—is limited by substantial interobserver variability. Agreement with the ACC/AHA classification was noted in only 58% of lesions in one series, with disagreement by two classification grades noted in almost 10% of lesions.2 Accordingly, rather than a composite score, description of individual morphological features may be more predictive of early and late outcome after PCI. Some ACC/AHA morphological features are associated with a complicated procedure (e.g., thrombus, saphenous vein graft [SVG] degeneration, angulated segments), whereas others are associated with an unsuccessful but uncomplicated procedure (e.g., chronic total occlusions, diffuse disease). The weighted kappa value for the interobserver reproducibility on the global score was 0.45, while the intraobserver weighted kappa value was 0.59.9 The SYNTAX score is reproducible (Δ 2.1%–3.4%).9,18 Other studies have also shown the importance of clinical variables in assessing early and late risk after PCI.19–22 Individual morphological criteria may also have individual prognostic value in assessing outcomes.
Irregular Lesions
With the advent of coronary stents, the prognostic importance of irregular lesions has been diminished substantially, although identification of an irregular plaque at angiography suggests the presence of an acute coronary syndrome and intracoronary thrombus. Semiquantitative and quantitative measurements of lesion irregularity were developed in the early 1990s to better characterize lesion morphology in patients with acute coronary syndromes, but these methods have not found clinical utility independent of other clinical risk factors. A novel technique of identifying plaque rupture (PR) defined any irregular lesion with ulceration, flap, or aneurysm on a qualitative angiogram as suspicious for PR. Intravascular ultrasound (IVUS)-detected PR and non-PR lesions were compared with the corresponding angiograms.23 A total of 224 distinct (ruptured or nonruptured) lesions were detected by IVUS in 65 patients; 49 of the 105 IVUS-detected nonculprit PRs were suspected on angiography.23 The positive and negative predictive values for correct angiographic diagnosis of PR were 96% and 61%, respectively.23 Proximal coronary location, wide cavity, and counterflow rupture were strong predictors of correct angiographic diagnosis, enabling four specific angiographic patterns to be identified using three-dimensional IVUS PR reconstruction.23
Lesion Calcification
Coronary artery calcium is an important marker for coronary atherosclerosis. Conventional coronary angiography has limited sensitivity for the detection of smaller amounts of calcium and is only moderately sensitive for the detection of extensive lesion calcium (60% and 85% sensitivity for three- and four-quadrant calcium, respectively).24 The presence of coronary calcification reduces the compliance of the vessel and may predispose to dissection at the interface between calcified plaque and normal wall after balloon angioplasty.25 The presence of coronary calcification also reduces the ability to cross chronic total occlusions; moreover, in severely calcified lesions, stent strut expansion is inversely correlated with the circumferential arc of calcium.24 Higher target lesion revascularization rates have been shown in patients treated with sirolimus-eluting stents who had lesion calcification compared with those who did not.26,27 Rotational atherectomy is the preferred pretreatment method in patients with severe lesion calcification, particularly ostial lesions; it facilitates the delivery and expansion of coronary stents by creating microdissection planes within the fibrocalcific plaque. Even with these contemporary methods, however, the presence of moderate or severe coronary calcification is associated with reduced procedural success and higher complication rates, including stent dislodgement. In less severely calcified lesions, no difference in restenosis rate was found after paclitaxel-eluting stent implantation in calcified versus noncalcified vessels.28 Calcification noted within SVGs is usually within the reference vessel wall rather than within the lesion and is associated with older grafts, insulin-dependent diabetes, and history of smoking.29 Calcified lesions were an independent predictor of stent thrombosis in one series.30
Degenerated Saphenous Vein Grafts
SVGs develop progressive degeneration over time, with 25% occluding within the first year after CABG31 and 50% developing occlusion within 10 years after surgery. Although coronary stents and, more recently, DESs11 reduce restenosis rates compared with balloon angioplasty, only embolic protection devices have reduced procedural complications.32,33 One exception may be in patients treated for in-stent restenosis (ISR), where embolic protection may not be required. Self-expanding stents made with expanded polytetrafluoroethylene (ePTFE) provide no additional advantage over noncovered balloon-expandable stents on the development of early complications or late restenosis.34 The risk for embolic complications appears to be related to both the degree of overall graft degeneration and the length of the lesion.35 Using angiographic assessments of the extent of graft degeneration and estimated volume of plaque in the target lesion, independent correlates of an increased 30-day rate of major adverse cardiovascular events (MACE) were more extensive vein graft degeneration (P < 0.0001) and bulkier lesions (larger estimated plaque volume, P < 0.0005). SVG lesions have been associated with a worse late outcome after PCI than native vessel lesions.4
Thrombus
Conventional angiography is relatively insensitive for the detection of coronary thrombus. The presence of angiographic thrombus, usually identified by the appearance of discrete intraluminal filling defects within the arterial lumen, is also associated with a higher albeit widely variable (6% to 73%), incidence of ischemic complications after PCI. A large thrombus burden is an independent predictor of stent thrombosis in patients with ST-elevation myocardial infarction (STEMI) treated with DESs.36 The primary complications related to PCI of thrombus-containing lesions are distal embolization and thrombotic occlusion, with the risk for complications with angiographic thrombus relating to the size of the coronary thrombus. Routine rheolytic thrombectomy provides no benefit in patients with AMI,37 although it may be useful for patients with a large thrombus burden. A number of aspiration catheters have been used in patients with AMI and large thrombus burden, but large-scale studies are lacking. Traditionally, the extent of coronary thrombus has been determined using the semiquantitative TIMI thrombus grade (TTG). A novel method of assessing intracoronary thrombus burden uses the discrepancy between luminal areas assessed with edge detection (ED) and videodensitometry (VD) as measured with the Cardiovascular Angiography Analysis System II.38 A good correlation between D-QCA and true occlusive volumes was found (y = 9.21 + 0.99x, correlation r = 0.996) with low intra- and interobserver variability.38 TTG also decreased in an in vivo model, but in 9 (47%) patients in whom TTG remained unchanged, D-QCA detected a reduction in thrombus burden (pre: 148.17 ± 154.03 mm3; post: 112.86 ± 117.82 mm3; P = 0.05).38 These results suggest that dual QCA appears to be a useful approach to the quantification of coronary thrombus volume, being more sensitive than the TTG in assessing changes in thrombus resulting from treatment strategies.38 The presence of thrombus remains an important predictor of outcome after PCI.3
Ostial Location
Ostial lesions are defined as those that begin within 3 mm of the origin of the coronary artery; they are classified as aorto-ostial and non-aorto-ostial. Balloon angioplasty of ostial lesions is limited by suboptimal procedural outcome, primarily owing to technical factors such as difficulties with guide-catheter support, lesion inelasticity precluding maximal balloon expansion, and early vascular recoil limiting the acute angiographic result. Debulking techniques such as directional and rotational atherectomy improve the compliance of the aorto-ostial lesion but have had limited effect on preventing late restenosis. Ostial lesion of the RCA and left circumflex artery have been associated with higher rates of target lesion revascularization (TLR) after DES placement.27 Coronary stenting, more recently with DESs, has become the default therapy for most aorto-ostial lesions. However, there are unique challenges of stent placement in the aorto-ostial location. These include protrusion of the stent into the aorta, thus precluding subsequent injection catheter engagement; compression; and avulsion of the stent struts into the aorta when new techniques, such as cutting balloon angioplasty, are used to treat ISR. Isolated non-aorto-ostial stenoses of the left circumflex and LAD39 and ostial side-branch bifurcation lesions are also effectively treated with DESs,40 but they pose unique challenges regarding vessel wall geometry, adequate ostial branch coverage (particularly if there is a narrow angle with the adjacent branch), and plaque shifting, causing compromise of the parent or adjacent branch vessels. Whereas stent protrusion into the parent vessel of less than 1 mm is usually well tolerated, stent protrusion to greater degrees precludes treatment of the parent branches.40 Stent fractures have been reported with more advanced stenting techniques used to treat the parent vessel and ostial side-branch stenoses.
Long Lesions
Lesion length may be estimated quantitatively as the “shoulder-to-shoulder” extent of atherosclerotic narrowing greater than 20%, although many clinicians estimate lesion length based on the identification of a “normal to normal” segment, which is usually longer than the length obtained with quantitative methods. Conventional balloon angioplasty of long lesions has been associated with reduced procedural success, particularly when the segment is diffusely diseased (i.e., <20 mm in length), primarily because of the more extensive plaque burden in long lesions. Stents improve late outcome compared with balloon angioplasty, but stent and lesion length remain the most important predictors of restenosis in the stent era.41 Coronary stents have been used to treat suboptimal angiographic results (“spot stenting”) and dissections after balloon angioplasty of longer lesions, although the “full metal jacket” stent approach to diffuse disease is associated with a higher recurrence rate in the absence of complete stent expansion, particularly in smaller vessels. Overlapping sirolimus-eluting stents provide safe and effective treatment for long coronary lesions.42 In one series, longer stented lesions were associated with stent thrombosis.30
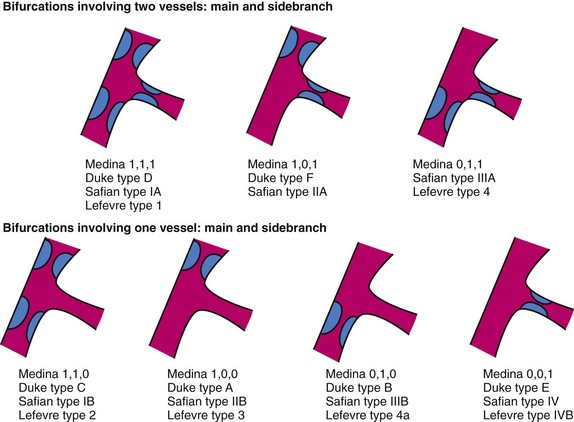
Bifurcation Lesions
The risk of side-branch occlusion in bifurcation lesions relates to the extent of atherosclerotic involvement of the side branch within its origin from the parent vessel, which ranges from 14% to 27% in side branches with ostial involvement. To accurately assess the risk of side-branch occlusion and avoid conflicting definitions of side-branch and ostial stenosis, a number of classification systems for bifurcation stenoses have been proposed (Fig. 56-1).43–46 Bifurcation lesions are predictors of stent thrombosis.47 One stent is preferable to stents in both the parent vessel and the side branch, because subacute thrombosis and restenosis remain higher in bifurcation disease treated with coronary stents in both branches.48 If two stents are planned for the parent vessel and side branch, a number of stenting techniques are possible, including simultaneous kissing stents, crush, coulotte,49 and T stenting. To date, the optimal technique has not been identified, although the use of DESs appears to reduce restenosis compared with bare metal stents. The origin of the side branch is the most common location of failure (recurrence) after bifurcation stenting.50 A number of dedicated bifurcation stents have been developed to provide adequate vessel coverage45 and side-branch access51 during stent deployment. Common to all of these strategies is a final “kissing” balloon inflation in the parent vessel and side branch.52 A specifically designed side-branch stent has also shown favorable initial results.53 A new bifurcation software (QVA-CMS V. 6.0 with the Bifurcation Module, MEDIS, Leiden, The Netherlands) has been developed to assess the severity of stenosis in patients with bifurcation stenoses using an analysis system that will allow examination of the bifurcation in three separate segments.54 A minimal cost algorithm is used to detect the contours of the proximal main vessel (PMV), distal main vessel (DMV), and sidebranch (SB) (QVA-CMS V. 6.0 with the Bifurcation Module, MEDIS, Leiden, The Netherlands). This new quantitative bifurcation analysis system can be consistently applied to the analysis of bifurcation lesions before and after angioplasty, with an intra- and interobserver reproducibility equal to or better than those of the conventional analysis system and also a significantly shorter analysis time.54
Total Occlusion
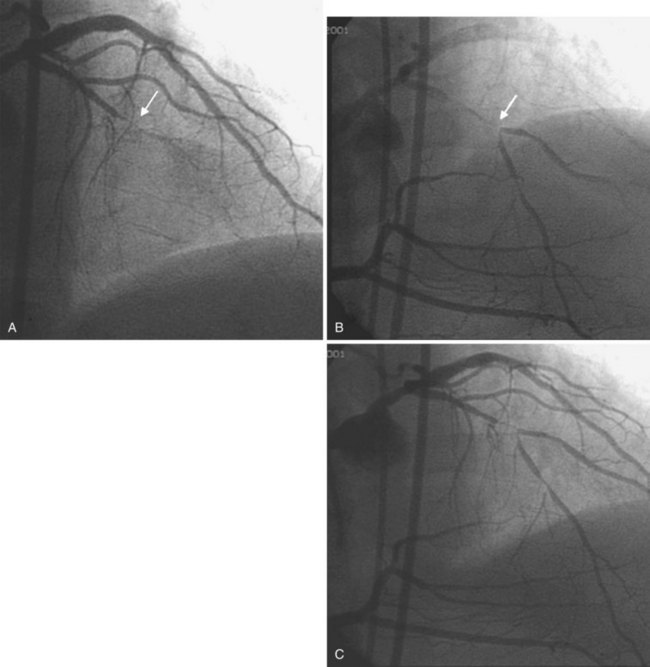
Coronary occlusion is identified as an abrupt termination of the epicardial vessel; anterograde and retrograde collaterals may be present and are helpful in quantifying the length of the totally occluded segment. Coronary occlusions are common findings55 and often lead to the decision to perform CABG rather than PCI in the setting of multivessel disease.56,57 The success rate for recanalization depends on the duration of the occlusion and on certain morphologic features of the lesion, such as bridging collaterals, occlusion length greater than 15 mm, and absence of a “nipple” to guide advancement of the wire. Although newer technologies and techniques have been used to recanalize refractory occlusions,58,59 better guidewires and wire techniques have accounted for much of the improvement in crossing success over the years.60 Simultaneous coronary injections are sometimes useful for identifying the length of the total occlusion (Fig. 56-2). Once the occlusion has been crossed, coronary stents, including DESs,61,62 have been used to provide the best long-term outcomes. A key component to the assessment of total occlusion is definition of the collateral grades that provide blood flow to the jeopardized myocardium.63 The Rentrop classification system includes Rentrop grade 0 (no filling), Rentrop grade 1 (small side branches filled), Rentrop grade 2 (partial epicardial filling of the occluded artery), and Rentrop grade 3 (complete epicardial filling of the occluded artery). Anatomical collaterals summarized by the 26 potential pathways were consolidated into four groups: septal, intra-arterial (bridging), epicardial with proximal takeoff (atrial branches), and epicardial with distal takeoff.64 Finally, the size of the collateral connection can be quantified as group 0 (no continuous connection between donor and recipient artery), group 1 (continuous threadlike connection ≤0.3 mm), or group 2 (continuous small, branch-like collateral through its course ≥0.4 mm).64 Three-dimensional (3D) imaging has been used for case planning in patients with chronic total occlusions.65 The novel CardiOp-B system for 3D reconstruction of the coronary vessels has been used in 302 angiographic images from 58 consecutive patients undergoing interventional treatment for 61 chronic total occlusion (CTO).65 The success rate of 3D reconstruction was 83%. When successful, these reconstructions led to a significant improvement in lesion analysis, especially at the stump area and/or missing segment. Importantly, in 92% of the successful 3D reconstructions, the artery path in the lesion area could be delineated. In most cases, 3D reconstruction of CTO can clearly image the stump area, delineate the lesion path, and provide enough information for the clinician to precisely calculate the severity of stenosis and lesion length. 3D reconstructions may serve as a useful tool for planning interventional procedures for CTO and improving their success rate.65
Angiographic Complications After Percutaneous Coronary Intervention
Although the frequency of angiographic complications during PCI has been reduced substantially with the use of coronary stents, untoward effects resulting from disruption of the atherosclerotic plaque and embolization of atherosclerotic debris, thrombus, and vasoactive mediators still occurs during 5% to 10% of PCI procedures (Table 56-4).
TABLE 56-4 Definition of Complications after Percutaneous Coronary Intervention
| Variable | Definition |
|---|---|
| Abrupt closure | Obstruction of contrast flow (TIMI 0 or 1) in a dilated segment with previously documented anterograde flow |
| Ectasia | A lesion diameter greater than the reference diameter in one or more areas |
| Luminal irregularities | Arterial contour that has a “sawtooth pattern” consisting of opacification but not fulfilling the criteria for dissection or intracoronary thrombus |
| Intimal flap | A discrete filling defect in apparent continuity with the arterial wall |
| Thrombus | Discrete, mobile angiographic filling defect with or without contrast staining |
| Dissection* | |
| A | Small radiolucent area within the lumen of the vessel |
| B | Linear, nonpersisting extravasation of contrast |
| C | Extraluminal, persisting extravasation of contrast |
| D | Spiral-shaped filling defect |
| E | Persistent luminal defect with delayed anterograde flow |
| F | Filling defect accompanied by total coronary occlusion |
| Length | Measure end-to-end for type B through F dissections |
| Staining | Persistence of contrast within the dissection after washout of contrast from the remaining portion of the vessel |
| Perforation | |
| Localized | Extravasation of contrast confined to the pericardial space immediately surrounding the artery and not associated with clinical tamponade |
| Nonlocalized | Extravasation of contrast with a jet not localized to the pericardial space, potentially associated with clinical tamponade |
| Sidebranch loss | TIMI 0, 1, or 2 flow in a side branch >1.5 mm in diameter that previously had TIMI 3 flow |
| Coronary spasm | Transient or permanent narrowing >50% when a <25% stenosis was previously noted |
TIMI, Thrombolysis in Myocardial Infarction.
* National Heart, Lung, and Blood Institute classification system for coronary dissection.
Coronary Dissection
Plaque fracture is an integral component of balloon angioplasty, although significant vessel wall disruption resulting in reduced anterograde flow and luminal compromise is a relatively uncommon occurrence (≈3%).66 The National Heart, Lung and Blood Institute (NHLBI) coronary dissection criteria categorize the severity of coronary dissection after PCI (Table 56-4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree