PULSE OXIMETRY SCREENING FOR DETECTING CONGENITAL HEART DEFECTS IN THE NEWBORN
Introduction
Congenital heart defects (CHDs) are the major birth defects in the United States (US) and the world. In the US, CHD is the leading cause of all infant deaths. At an estimated rate of 0.8% live births,1 about 40,000 babies are born with a CHD in the United States. Globally, it is estimated that over 1 million babies are born with a CHD every year. About 100,000 of them will not live to see their first birthday, and thousands more will die before they reach adulthood. In the US, the prevalence of CHD is steadily increasing. The prevalence increased from 6.2 per 1000 in 1968–1995 to 9.0 per 1000 births in 1995–1997,2 which amounts to about 7200 babies annually. This increase is thought to be most likely due to the improved diagnosis using new technologies of fetal and postnatal echocardiography. The cost associated with the management of CHD is also a major economic burden to families with a child with CHD.3
Until recently, the diagnosis of CHD was based on clinical findings, including cyanosis, abnormal heart sounds, and cardiac murmur, supported by laboratory investigative tools, for example, ECG and chest radiography. Echocardiograms have become readily available during the last 2 decades; they are within the domain of pediatric cardiologists. However, they are not accessible to primary care physicians caring for the newborn in a community hospital setting. Although antenatal diagnosis of fetal conditions including fetal cardiac lesions is now common, the availability of fetal echocardiography is limited to tertiary care hospitals. Where available, fetal diagnosis of CHD is feasible only in the hands of experts. Therefore, there is increasing interest in developing a simple screening tool for the diagnosis of CHD, particularly the asymptomatic critical CHD, at the earliest opportunity.
The introduction of pulse oximetry in the newborn to assess oxygen saturation noninvasively has opened new avenues to detect critical CHD in the immediate neonatal period. In this chapter, the author will summarize the rationale for using pulse oximetry in the neonatal period for the detection of CHD in general and critical CHD in particular. We will also discuss the current suggested protocol for implementing pulse oximetry for universal neonatal screening of the newborn for detection of CHD and critical CHD.
Definition of CHD and Critical CHD
During the last 3 to 4 decades, there has been a steady improvement in the outcome of infants with CHD, thanks to early diagnosis and advances in cardiac surgery. CHD is the most common disease among congenital diseases, accounting for 8 to 12 per 1000 live births4; about half of the CHDs are asymptomatic at birth but may manifest within days after birth. Early diagnosis is important because delayed diagnosis results in development of acidosis, shock, and a greater risk of mortality. Anatomically, CHD is defined as the “presence of gross structural abnormality of heart or intrathoracic great vessels that is actually or potentially of functional significance.”5 A list of critical CHDs as defined by the US Centers for Disease Control and Prevention (CDC) is given in Table 9.1.
Table 9.1. Pulse oximetry screening is most likely to detect the following 7 critical CHDs
|
Other heart defects can be just as severe as the main screening targets (see Table 9.1) and also require treatment soon after birth. However, pulse oximetry screening may not detect these heart defects as consistently as the above 7.
It is recognized that asymptomatic newborns with any one of these lesions, unless detected early by other means in the neonatal period, would have a poor outcome, including death. It is in this context that the use of pulse oximetry has gained importance.
The early report by Hoke et al6 is probably the first one to show that CHD can be detected by pulse oximetry, a simple, noninvasive method of measuring oxygenation. Since then, a large body of evidence has developed showing that routine pulse oximetry in newborns after 24 hours of age is highly useful in identifying CHDs before they become symptomatic.7,8 In this chapter, we review the available literature on the use of pulse oximetry in the screening of CHD and critical CHD in the immediate neonatal period, and discuss the current recommendations for universal screening of newborns for critical CHD.
Prevalence and Incidence of CHD and Critical CHD
Congenital heart anomalies are one of the most common causes of death in infancy. In advanced countries, they are the leading cause of death among infants, accounting for 40% of deaths from all congenital anomalies. These deaths also account for 3% to 7.5% of overall infant deaths.8 The term CHD includes a wide variety of cardiac defects consisting of defects with no clinical importance and those with critical functional abnormality. Hoffman9 defined the critical CHD as those conditions to which infants without surgical interventions would succumb; the list is similar to those defined by CDC as critical CHDs and given in Table 9.1.
The increasing incidence of CHD is attributed to an increased level of diagnosis using modern echocardiographic technology. As stated earlier, considerable progress has been made in the survival of infants with CHD during the last half of the last century. The improvements in the outcome are mainly due to advances in surgical techniques. Oster et al10 conducted a retrospective analysis of survival of CHD infants born with CHD during 1979 to 2005. They developed Kaplan–Meier survival probability curves for 12 critical CHDs and compared with those of infants without CHD. They showed that 1-year survival of infants with CHD improved from 75% to 97% for infants with noncritical CHD. One-year survival of infants with critical CHD improved from 67.4% in 1979–1993 to 82.5% survival in 1994–2005 (P < 0.001). In spite of these advances, not all CHDs are diagnosed within the immediate neonatal period.
The mortality rate from CHD is also reported to have decreased by 42%, with postoperative mortality of only 3.7%.11 Moreover, it is estimated that 80% of babies born with CHD survive up to 16 years of life. However, late detection of critical CHD has a very unfavorable outcome.12,13 Current hospital practices are aimed at early discharge to shorten hospital stay and decrease the associated healthcare cost. These factors increase the chance of missing the diagnosis of CHD. Thus, there is an increasing interest on early and accurate diagnosis of critical CHD that will lead to timely surgical treatment. Currently available methods of diagnosis of CHD, their pitfalls, and the emerging advantage of using pulse oximetry in the immediate neonatal period before discharge are described below.
Current Methods of Diagnosis of CHD
Physical Examination of the Newborn
Prior to the availability of fetal echocardiography, the presence of CHD in the newborn was diagnosed only after the birth of the infant. Physical examination has been the main approach for the diagnosis of CHD in clinical practice. In hospitals without the availability of specialists and in resource-poor countries, clinical examination by physicians and healthcare personnel is the only available tool. However, experience shows that physical examination alone may miss the diagnosis of CHD at discharge of the baby in 30% to 50% cases.9 The presence of cyanosis and presence of a murmur are the telling signs of a CHD in the newborn.
Clinical diagnosis is more accurate in the hands of an experienced pediatric cardiologist. However, for many, mild cyanosis is difficult to detect with naked eye, leading to failure of diagnosis of CHD. Furthermore, not all CHDs present with cyanosis in the early stages, especially those dependent on the ductus.14 These are the pitfalls of clinical diagnosis. Infants who have a missed early diagnosis of CHD, particularly with critical CHD, return to hospitals soon after discharge in shock and acidosis and have a high incidence of mortality and morbidity even if surgical interventions are made available at that time.
The presence of cardiac murmur is another important clinical sign of CHD. Again, in this instance, diagnosis of CHD is most accurate in the hands of an experienced physician, for example, the pediatric cardiologist. Clinical diagnosis is fraught with large discrepancies between the finding of a cardiac murmur and diagnosis of CHD in the newborn. The incidence of cardiac murmur during the first week of life ranges between 0.6% and 4.2%.15,16 Detection of murmurs also depends on clinical expertise, and the age at which the newborn is examined. It is well recognized that 50% of infants with CHD do not exhibit murmur in the immediate postnatal period. These are the pitfalls of dependency on cardiac murmurs in diagnosing CHD and critical CHD.
Echocardiography
Postnatal echocardiography has become the gold standard for the diagnosis of CHD in the postnatal period.12 However, even echocardiographic findings may provide high rates of false-positive reports and are expensive.16,17 Thus, echocardiograms have several limitations. It is best only in the hands of pediatric cardiologists and thus not readily available with other physicians. Although echocardiogram is the gold standard method of establishing CHD in the postnatal period, it is not a suitable candidate for universal screening for detecting CHD in the newborn.
Antenatal Diagnosis
Antenatal diagnosis of CHD is the most recent advance in perinatal medicine. Fetal ultrasound is a routine procedure in prenatal practice. Inability to demonstrate a 4-chamber view of cardiac image allows the sonographer to identify cardiac defects in the fetus. Fetal ultrasound is routine in advanced countries and is increasingly used even in developing countries.
Fetal ultrasound imaging is offered to pregnant women during the period of 18 to 22 weeks.18 Diagnosis of CHD at this stage allows the perinatologists/neonatologists/cardiologists to provide appropriate counseling to parents. Even with these fetal ultrasound techniques, the antenatal detection of CHD is only 20% to 50% and the detection of critical CHD is only 50%.19 Fetal echocardiography, as noted, is limited to the availability of expert obstetricians/perinatologists and is not without cost for universal screening.
Pulse Oximetry in the Immediate Neonatal Period
Since the availability of technology of pulse oximetry for use in neonatal monitoring,6 it has become a simple yet an efficient method of monitoring oxygenation in the neonatal intensive care units across the globe; more so in developed countries than in developing countries. Investigators quickly realized the potential of using a simple, noninvasive method of pulse oximetry to detect CHD in the newborn. The underlying premise of using pulse oximetry in detecting cyanotic congenital heart disease is based on the fact that there is right-to-left intracardiac shunting causing systemic arterial desaturation. In addition, a differential oxygenation in the preductal upper limb compared with postductal lower limbs because of the right-to-left shunting of blood across the ductus also indicates the presence of CHD.
Numerous studies have explored the value of pulse oximetry in the early detection of CHD with right-to-left shunting in asymptomatic newborns.6–9 Studies also explored whether a combination of clinical examination and pulse oximetry will increase the detection of CHD in the immediate neonatal period in an otherwise normal newborn.
Criteria for Developing a Screening Test
Once the value of the use of pulse oximetry in detecting critical CHD was established in 1995,6 pulse oximetry was considered as a valuable screening tool to detect congenital cardiac disease in the newborn.
Meberg et al,20 and later Ewer et al,8 conducted studies in large newborn populations to establish the usefulness of pulse oximetry to detect CHD and critical CHD. The findings of these studies led to support for the concept of using pulse oximetry as a universal screening for CHD in the newborn period. In making such a recommendation, one must consider whether the pulse oximetry test meets the criteria for universal screening. The World Health Organization (WHO) has developed specific criteria21 (Table 9.2) for validating a screening test for universal screening programs. Basically, it requires that there is a sufficient knowledge of the disease to be tested. The disease to be detected should be of considerable importance as a public health issue. The natural history of the disease should be well understood with respect to latency and early symptomatic states. The test also must be a suitable test for the intended disease to be detected. It also should be acceptable to all population; case finding must be a continuous process. From the clinical aspects of treatment, there should be an acceptable treatment regimen. Finally, the facilities for diagnosis and treatment must be accessible to the general population.
Table 9.2. World Health Organization (Wilson–Jungner) criteria for appraising the validity of a screening program21
Knowledge of the disease
|
Knowledge of the test
|
Treatment for the disease
|
| Cost consideration |
| Cost of case finding and treatment should be balanced in relation to possible total cost of treatment balanced in relation to possible total cost of treatment. |
Adopted from Kerruish NJ and Robertson SP21
In terms of public health policy, the cost of population screening must be economically balanced between the cost of screening and diagnosis and treatment. Experience from initiating several other newborn screening tests shows that the screening for phenylketonuria (PKU) and other genetic disorders in the neonatal period have all been found cost effective in developed countries. Hoffman9 argued that pulse oximetry screening test for detecting critical CHD meets all the above-mentioned WHO criteria. In fact, it was observed that critical CHD is far more frequent than many other metabolic disorders for which a screening test is currently mandatory. Therefore, universal pulse oximetry screening to detect critical CHD is equally reasonable.
Case for Pulse Oximetry for Universal Screening for CHD
The earliest large-scale studies of screening for CHD came from Sweden, Norway, and the United Kingdom (UK). These and other studies have been reviewed extensively by Ewer,8 Knowles12 and their associates in preparing the technical reports on pulse oximetry in the UK, and by Thangaratnam et al7 in a study of meta-analysis of published reports. Their observations are summarized below.
Technical Report from the UK
To establish the validity of pulse oximetry screening in detecting CHD, the investigators screened 20,055 newborns in 6 centers across the UK.8 They identified 53 cases of major CHDs: 24 critical and 29 serious. This gave a prevalence of 2.6 per 1000 live births. The sensitivity was 75.0% for CHD and 49.1% for all major CHDs. Of these, 23 cases were diagnosed antenatally. Exclusion of these cases from the analysis gave a sensitivity of 58.1% for critical CHD and 28.6% for major CHD. There was a false-positive rate of 0.86% (1/119). The false-negative rate was 1.4 per 1000 live births.
Among the 169 false-positive cases, 6 infants had significant but not major CHD, and 40 cases were of respiratory or infectious nature requiring medical intervention. Among the false-negative cases, 27 babies with major CHDs were missed. Parents were generally well satisfied with the screening. Mothers with false-positive cases were no more anxious than those with true negative cases. The incremental cost effective ratio of pulse oximetry and clinical examination compared with clinical examination alone was approximately £24,900 per timely diagnosis in a population where the antenatal diagnosis of CHD already exists. The study concluded that pulse oximetry is a simple, safe, and feasible test that is acceptable to parents and staff that added value to the existing screening. It is also cost effective in detecting critical CHD; the test is also helpful in detecting other respiratory and infectious diseases.
Meta-Analysis of Published Reports
Thangaratnam et al7 reviewed 552 studies related to oximetry screening for detecting CHD. Of these, they identified 13 studies eligible for meta-analysis to determine the accuracy of pulse oximetry to detect critical CHD in asymptomatic newborns. Thirteen studies conducted during the period of 2002 to 2011 included a total of 229,421 babies. Of the 13 reports included in the meta-analysis, all studies defined the inclusion criteria and had description of and the representative range of patients; appropriate and independent reference standards were defined. Owing to several details and use of standard reference, authors considered these studies to be strong and credible to be included in their meta-analysis.
Table 9.3 below shows accuracy estimates of 13 studies included in the meta-analysis and the effect of different variables. The study established specificity and sensitivity of pulse oximetry screening to detect CHD. They also looked at different variables related to probe application. Seven of the 13 studies used foot only, whereas in 6 of the 13 studies, both right hand and one foot were used for application of the probe. Six of the 13 studies tested pulse oximetry before 24 hours of age. In 7 of the 13 studies, infants were tested after 24 hours of age. Four of the 13 studies included cases that had antenatal diagnosis of CHD. They also calculated true positives, false positives, true negatives, and false negatives. The meta-analysis concluded that overall sensitivity was 76.5% with a narrow 95% CI of 67.7 to 83.5. The false-positive rate was 0.14% (95% CI of 0.06–0.33). False positives were also higher when foot and right hand were tested simultaneously compared with testing foot only. False positives were lower when tested after the age of 24 hours. False-positive rates did not differ significantly irrespective of whether one or 2 sites were used (P < 0.6). Inclusion of cases with antenatal diagnosis increased false-positive results. Authors concluded that “there is compelling evidence for the introduction of pulse oximetry as a screening method in clinical practice.”
Table 9.3. Overall sensitivity and false-positivity rates of pulse oximetry values
| Parameter studied | Sensitivity (95% CI) | False-positive rate (95%CI) |
| Overall estimate | 76.5 (67.7–83.5) | 0.14 (0.06–0.33) |
| Timing of test (age ) | ||
| <24 hours | 84.8 (69.8–93.1) | 0.50 (0.29–0.86) |
| >24 hours | 77.5 (61.8–88.0) | 0.05 (0.02–0.12) |
| Site of probe application | ||
| Foot and right hand | 70.0 (54.9–81.7) | 0.19 (0.04–0.89) |
| Foot only | 80.2 (69.5–87.8) | 0.12 (0.04–035) |
| Positive antenatal screen for CHD | ||
| Excluded | 76.7 (66.4–84.5) | 0.08 (0.03–0.19) |
| Included | 88.1 (62.6–97.0) | 0.73 (0.50–1.05) |
Modified from Thangaratnam.7 The table gives overall sensitivity and false-positivity rates of pulse oximetry values in detecting critical CHD. It also shows differences in sensitivity and false positivity of testing according to the time of testing, placement site, and antenatal fetal echocardiographic screening. See the text for details.
Cost Effectiveness of Screening and the Impact of False-Positive Results on Parents
Hoffman9 concluded that cost of screening for CHD in the neonatal period was considerably lower than the cost of screening tests for metabolic diseases. He analyzed the cost benefits of pulse oximetry screening to detect critical CHD in four scenarios.
True-Positive Screening Results
There is a great advantage of detecting a true-positive case on screening for CHD. He estimated that the cumulative incidence of congenital metabolic disorders in the US is around 1600/million births. With annual births of 4 million, total cases amount to 6400/year. On the other hand, the incidence of CHD is 4000/million births or 16,000/year. Of these, 1200/million may be missed diagnoses without screening. He estimated that the cost of pulse oximetry screening may account for approximately $10,000 per asymptomatic critical CHD case.
True Negatives
In the case of true-negative finding, there is no cost beyond the primary screening. However, there is an invaluable advantage for parents and the physician in knowing that critical CHD has not been missed.
False Negatives
Missing a critical CHD could be a serious problem. However, in the Norwegian20 study, the rate of false-negative finding was only 0.008%. Others have noted that those false negatives are much higher without screening.22,23 In the meta-analysis of 13 studies published between 2002 and 2011, Thangaratnam et al7 found the likelihood of false negatives to be 0.24% with 95% CI of 0.17 to 0.33.
False Positives
Great concern has been expressed regarding false-positive results of screening for CHD. The concerns are the need for unnecessary cost of additional investigations and emotional cost to families. However, the false-positive rate increased to 0.035% if screening is carried out after 24 hours of age.16 In the meta-analysis data, the false-positive rate was 0.14%.
It is becoming increasingly evident that the pulse oximetry screening test to detect critical CHD is cost effectiveness.7,8,12 Some of the relevant studies are summarized below.
Roberts et al24 were the first group to study the cost effectiveness of oximetry screening of newborns to detect CHD. They have shown that the addition of pulse oximetry to clinical examination increased the detection of more babies with CHD, although it increased the cost. However, the probability of the screening test being cost effective was found to be greater than 90%. Similar conclusions were reached by Ewer et al after their studies in the UK.8
Knowles et al12 studied the cost effectiveness of pulse oximetry in detecting CHD in comparison to echocardiography. Even though echocardiography was associated with greater detection of CHD, it was also very expensive. Thus, pulse oximetry was found to be cost effective in detecting CHD. In the study by Roberts et al,24 investigators compared clinical examination with clinical examination plus pulse oximetry. They found that the combination of clinical examination and pulse oximetry was cost effective in detecting CHD. The incremental cost saving by addition of pulse oximetry to clinical examination was about $38.74. From their analysis, they concluded that the probability of pulse oximetry screening being cost effective was greater than 90%.
Peterson et al25 reported the first cost–benefit analysis of pulse oximetry screening in the US. Based on the current number of annual births of 3.8 million in the US, they calculated that 1189 additional cases of critical CHD will be diagnosed with pulse oximetry screening, and it will avert 20 deaths. However, screening will miss 345 babies with CCHD with pulse oximetry screening because it is not 100% sensitive. Moreover, the test would yield 1975 false-positive cases. Practitioners need to be aware of these shortcomings of pulse oximetry screening. Costwise, the authors conclude that pulse oximetry will be cost effective and cost saving, using conventional thresholds of oxygen saturation.
Screening Time: As Little As 3.5 Minutes13
As discussed earlier, there is a great concern regarding the rate of false-positive tests of pulse oximetry screening because of unnecessary expensive testing incurred by parents and additional stress of dealing with false-positive results, when the infant actually does not have CHD. In a meta-analysis of 13 large studies, Thangaratnam et al7 found the rate of false positives in pulse oximetry screening tests to be only 0.14% with 95% CI of 0.06% to 0.33%. Studies in UK investigators8 found that there was no evidence for the hypothesis that mothers were any more anxious with false-positive results of their babies than mothers who had true-negative results. They found that the degree of stress from false-positive results was related to other factors: levels of baseline stress, anxiety, depression, and understanding the disease and available treatment choices.
Recommendations for Universal Screening and Implementation in the US
Although pulse oximetry as a screening tool for critical CHD has been endorsed by the American Heart Association,26 the American Academy of Pediatrics,16 and the March of Dimes,27 there are several steps needed to adopt the test for universal screening in the US. Bradshaw et al28 provided details of the process of establishing a new test for “Universal Screening.” The information is highly useful for the readers. Any new screening test to be added to the list of universal screening requires that the Human and Health Services (HHS) advisory committee on heritable diseases in newborn and children identifies and evaluates the tests. The committee after satisfactory evaluation recommends the new test to be added to the recommended uniform panel. The recommended screening tools undergo further evaluation by the Interagency Coordinating Committee before final endorsement by the HSS. Finally, the US Department of HHS adds the test to the list of recommended tests. Pulse oximetry in the immediate neonatal period as a screening tool to detect critical CHD was added to the list in September 2011.29 The speed and ease of this endorsement was based on the fact that screening can be carried out with the existing system without additional cost to the institution. For example, it was found on a survey that 100% of hospitals surveyed in Wisconsin had pulse oximetry units. Nursing staff performed pulse oximetry, and 74.4% of hospitals surveyed also had access to same-day neonatal echocardiography. The survey findings suggested that screening can be easily initiated in community hospitals.30,31 Eighty-eight of 99 hospitals surveyed (89.9% of hospitals covering 95% of states annual births) responded; 100% of the hospitals had availability of pulse oximetry. There were variations in brands used. After initiating the training, the trainer program’s average time required to apply the sensor was 3.5 minutes. No additional staff was required. Barriers were reported only in 2.4% of the cases.
In spite of the ease of administration of the test without additional cost to hospitals, it is noteworthy that the new screening tool to detect critical CHD was only recommended, but not made mandatory with/in newborn screening tool. Not all states have adopted the recommendation, although the number of states initiating the screening for critical CHD is steadily increasing. The State of Maryland was the first state to legislate development of a plan.32 New Jersey was the first state to legislate implementing the pulse oximetry screening.33 Since then, 26 states have adopted the policy of screening for critical CHD in the neonatal period.
Approach to the Diagnosis and Management of Results of Universal Screening for Critical CHD
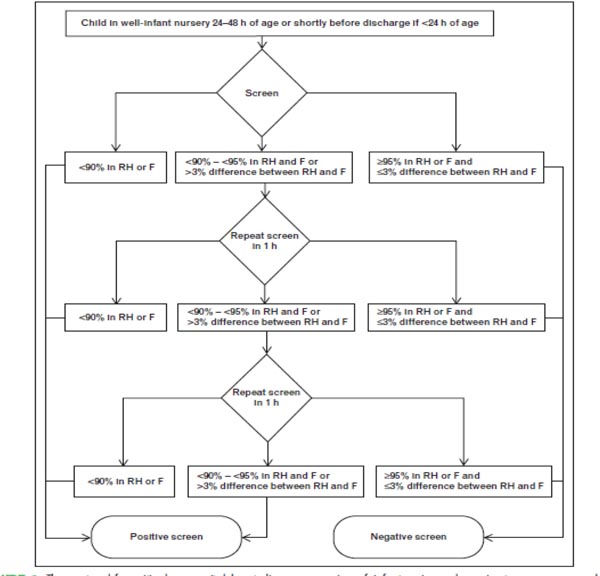
The diagram (Figure 9.1) and Table 9.4 provide an algorithmic approach to universal pulse oximetry in detecting the asymptomatic critical CHD in the newborn.34 The test is to be administered to infants of >24 hours of age. The screening tools must be motion tolerant and those approved by the Food and Drug Administration (FDA). The algorithm is based on the application of probe both on the right hand and on one of the feet. The clinician should follow the directions given in the algorithm.
Figure 9.1. An algorithm for the detection of critical CHD in the newborn using pulse oximetry screening at >24 hours of age.34 Positive screen or failing suggests that the baby has critical CHD. Negative screen excludes critical CHD. However, CoA cannot be ruled out. See also Tables 9.4A and 9.4B, for interpretation and management of babies with pulse oximetry values <90%, and those between 90% and 95%.
If pulse oximetry shows that oxygen saturation is <90% at any time, or <95% on both sites, or >3% difference between the sites for three measurements separated by 1 hour, the test is considered positive for critical CHD. If the infant shows >95% oxygen saturation, the test is deemed negative, that is, no critical CHD. As stated earlier, these values have lower false positives if infants are tested at >24 hours of age. The oxygen saturation cut-off points for the detection of critical CHD must be adjusted for infants born and tested at higher altitudes. The values should be lower than infants born and tested at the sea level.35,36
Table 9.4. Management of cases after pulse oximetry screening
A. Cases that Fail on First Screening Test. SpO2 <90%
B. Cases in which Readings Are Between 90% and 94% and Preductal and Postductal Differences Are Between 90% and 94% Saturation on Subsequent Hourly Tests Repeated Twice
|
SOME CAVEATS:
Recognize that the screening algorithm is a guide only. Clinicians should make decisions based on clinical judgment alone. A negative screen does not always rule out a CHD (noncyanotic coarctation of aorta [CoA])
Adopted from Alabama Department of Health guidelines for implementing pulse oximetry screening for critical congenital heart disease, March 2012.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree