Pulmonary Vasculitis
Vasculitis Overview
GENERAL PRINCIPLES
• This chapter discusses the pulmonary features of systemic vasculitides, primarily focusing on the antineutrophil cytoplasmic antibody (ANCA)-associated small-vessel vasculitides granulomatosis with polyangiitis (GPA) (formally known as Wegener granulomatosis), microscopic polyangiitis (MPA), and Churg–Strauss syndrome (CSS). Each of these vasculitides will be considered in separate sections in this chapter.
• Other vasculitides with pulmonary manifestations will be described under special considerations.
Definition
• Systemic vasculitides feature inflammatory leukocytes damaging the walls of blood vessels. This damage can lead to vessel wall inflammation and downstream tissue ischemia.
• Vasculitis is a pathologic finding, not a diagnosis. Clinicians must determine the cause of the vasculitic condition.
Classification
• Vasculitides may be classified as primary versus secondary processes. Primary vasculitides are further classified by the size and type of involved blood vessels.
• Primary vasculitis occurs in the absence of an underlying illness and without identifiable etiology.
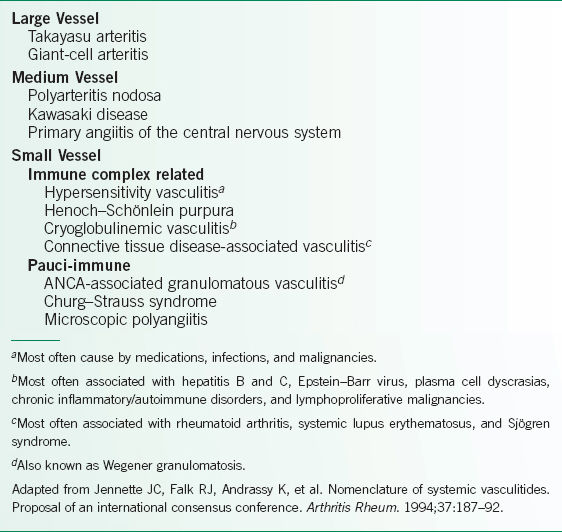
 Several classification schemes have been described, but the most commonly utilized one is derived from the 1993 Chapel Hill Consensus Conference, so-called “CHC criteria”.1
Several classification schemes have been described, but the most commonly utilized one is derived from the 1993 Chapel Hill Consensus Conference, so-called “CHC criteria”.1
 While vasculitis may often affect vessels of more than one size, the CHC criteria organized the vasculitides based on the size of the vessels primarily affected (Table 20-1).1 The small-vessel vasculitides are further subdivided into ANCA positive or ANCA negative.
While vasculitis may often affect vessels of more than one size, the CHC criteria organized the vasculitides based on the size of the vessels primarily affected (Table 20-1).1 The small-vessel vasculitides are further subdivided into ANCA positive or ANCA negative.
• Secondary vasculitis occurs in the presence of an underlying condition, such as the capillaritis seen in systemic lupus erythematosus (SLE) or virus-induced vasculitis.
Epidemiology
• Although the primary systemic vasculitides are rare and epidemiologic studies have been difficult in the setting of evolving classification systems and nomenclature, the frequency of vasculitic diagnoses has been increasing, possibly because vasculitic syndromes are more readily recognized.
• At an annual incidence of 13 cases/million adults, giant-cell arteritis (GCA) represents the most common vasculitis. GCA is followed in annual incidence by rheumatoid arthritis (RA)-associated vasculitis (12.5 cases/million), GPA (8.5 cases/million), MPA (2.4 cases/million), CSS (2.4 cases/million), and Henoch–Schönlein purpura (1.2 cases/million).2
TABLE 20-1 CLASSIFICATION OF VASCULITIS
DIAGNOSIS
• Clinicians should consider a diagnosis of vasculitis in patients whose clinical presentation includes systemic symptoms (e.g., fatigue, weakness, fever) as well as evidence of organ dysfunction (e.g., renal, neurologic, pulmonary). As the clinical manifestations of the vasculitides are quite variable and overlap with many other disorders, a thorough history and physical examination is essential to aid in the diagnosis of vasculitis. The clinical presentation of the small-vessel ANCA-associated vasculitides will be discussed in each of their respective sections found below.
• Pulmonary manifestations of the systemic vasculitides may range from shortness of breath due to mild upper respiratory tract symptoms to pulmonary failure resulting from devastating alveolar hemorrhage. While the lungs may not represent the only involved organ system for many cases of vasculitis, respiratory symptoms often motivate these patients to seek medical attention.3
• Basic laboratory tests for the vasculitides should include serum creatinine, liver function tests, complete blood count, muscle enzyme tests, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), viral hepatitis and HIV serologies, urinalysis, and urine toxicology screen. More specific tests may include antinuclear antibody (ANA, for lupus), serum complement levels (depressed in mixed cryoglobulinemia and lupus), and ANCA (often directed against proteinase 3 in GPA and against myeloperoxidase (MPO) in MPA).
• Certain procedures are often crucial for the diagnosis of vasculitides. Specifically, tissue biopsy can definitively establish the presence of vasculitis. Arteriography is useful for vasculitides affecting large- and medium-sized blood vessels, as in arch angiography for Takayasu arteritis. Similarly, mesenteric or renal artery angiography can assist in patients suspected of having polyarteritis nodosa (PAN).
TREATMENT
While the treatments for ANCA-associated vasculitis will be discussed in their respective sections, management of most vasculitides generally involves corticosteroids, typically in combination with other immunosuppressive agents. Other considerations in management specific to a particular vasculitis will be discussed with that particular syndrome.
SPECIAL CONSIDERATIONS
• Primary large-vessel vasculitides
 GCA or temporal arteritis represents the most common vasculitis among Caucasians, predominantly affects the elderly, and classically involving the extracranial branches of the carotid artery.
GCA or temporal arteritis represents the most common vasculitis among Caucasians, predominantly affects the elderly, and classically involving the extracranial branches of the carotid artery.
 Respiratory symptoms such as cough, hoarseness, or throat pain represent the initial complaint in up to one-quarter of patients, although CXRs and pulmonary function testing may be normal.
Respiratory symptoms such as cough, hoarseness, or throat pain represent the initial complaint in up to one-quarter of patients, although CXRs and pulmonary function testing may be normal.
 In a patient with suspected GCA, fulfilling three of the following five criteria is associated with >90% sensitivity and specificity for the diagnosis of GCA: age ≥50 years at disease onset, new-onset localized headache, tenderness or decreased pulse of temporal artery, ESR >50, and a biopsy with necrotizing arteritis with a predominance of mononuclear cells or a granulomatous process with multinucleated giant cells.4
In a patient with suspected GCA, fulfilling three of the following five criteria is associated with >90% sensitivity and specificity for the diagnosis of GCA: age ≥50 years at disease onset, new-onset localized headache, tenderness or decreased pulse of temporal artery, ESR >50, and a biopsy with necrotizing arteritis with a predominance of mononuclear cells or a granulomatous process with multinucleated giant cells.4
 A common variant of GCA is large-vessel GCA, which manifests as arm claudication, pulselessness, aortic aneurysms, or aortic insufficiency.
A common variant of GCA is large-vessel GCA, which manifests as arm claudication, pulselessness, aortic aneurysms, or aortic insufficiency.
 Treatment: long-term prednisone treatment (9–12 months) usually leads to symptom resolution in GCA.5 Optimal steroid dosing is not precisely known.
Treatment: long-term prednisone treatment (9–12 months) usually leads to symptom resolution in GCA.5 Optimal steroid dosing is not precisely known.
 Takayasu arteritis affects the aorta and its major branches, and is classically described in young Asian females.
Takayasu arteritis affects the aorta and its major branches, and is classically described in young Asian females.
 Manifestations can include mild pulmonary hypertension, fistula formation between branches of the pulmonary artery and bronchial arteries, and/or nonspecific inflammatory interstitial lung disease.
Manifestations can include mild pulmonary hypertension, fistula formation between branches of the pulmonary artery and bronchial arteries, and/or nonspecific inflammatory interstitial lung disease.
 CT or MRI angiography demonstrates pulmonary artery stenoses and occlusion in nearly half of patients.
CT or MRI angiography demonstrates pulmonary artery stenoses and occlusion in nearly half of patients.
 Diagnostic criteria: 3 of 6 is associated with a sensitivity of 90% and specificity of 98% for the diagnosis of Takayasu arteritis: age ≤40 years at disease onset, extremity claudication, decreased pulsation of at least one brachial artery, systolic blood pressure difference ≥10 mm Hg between the arms, bruit over a subclavian artery or abdominal aorta, and arteriographic narrowing or occlusion of the aorta or its major branches not due to other causes.6
Diagnostic criteria: 3 of 6 is associated with a sensitivity of 90% and specificity of 98% for the diagnosis of Takayasu arteritis: age ≤40 years at disease onset, extremity claudication, decreased pulsation of at least one brachial artery, systolic blood pressure difference ≥10 mm Hg between the arms, bruit over a subclavian artery or abdominal aorta, and arteriographic narrowing or occlusion of the aorta or its major branches not due to other causes.6
 Treatment: Glucocorticoids and immunosuppression are used for initial treatment, while methotrexate or vascular bypass are options for severe or refractory cases.
Treatment: Glucocorticoids and immunosuppression are used for initial treatment, while methotrexate or vascular bypass are options for severe or refractory cases.
• Primary medium-vessel vasculitides
 PAN is a necrotizing systemic vasculitis affecting both medium and small muscular arteries.
PAN is a necrotizing systemic vasculitis affecting both medium and small muscular arteries.
 Associated with hepatitis B infection.
Associated with hepatitis B infection.
 Can present with skin nodules, mononeuritis multiplex, orchitis, and mesenteric artery involvement, but does not typically cause glomerulonephritis or vasculitis of the arterioles, capillaries, or venules.
Can present with skin nodules, mononeuritis multiplex, orchitis, and mesenteric artery involvement, but does not typically cause glomerulonephritis or vasculitis of the arterioles, capillaries, or venules.
 Although pulmonary involvement is extremely rare in PAN, it can occur as alveolar hemorrhage or diffuse alveolar damage, manifesting as diffuse interstitial or patchy alveolar infiltrates, even when hepatitis B virus related.7
Although pulmonary involvement is extremely rare in PAN, it can occur as alveolar hemorrhage or diffuse alveolar damage, manifesting as diffuse interstitial or patchy alveolar infiltrates, even when hepatitis B virus related.7
 Treatment: high-dose corticosteroids and, if necessary, additional immunosuppressants.
Treatment: high-dose corticosteroids and, if necessary, additional immunosuppressants.
 Kawasaki disease (KD), while primarily a medium-vessel vasculitis, can also affect large and small blood vessels.
Kawasaki disease (KD), while primarily a medium-vessel vasculitis, can also affect large and small blood vessels.
 Usually seen in children, this vasculitis has a predilection for the coronary arteries, and may be associated with a mucocutaneous lymph node syndrome.
Usually seen in children, this vasculitis has a predilection for the coronary arteries, and may be associated with a mucocutaneous lymph node syndrome.
 While pulmonary symptoms are not among the criteria for diagnosis, pulmonary involvement can occasionally occur in KD, and may be misinterpreted as atypical pneumonia or unresolving pneumonia, with findings ranging from subclinical interstitial micronodular infiltrates to larger inflammatory pulmonary nodules.
While pulmonary symptoms are not among the criteria for diagnosis, pulmonary involvement can occasionally occur in KD, and may be misinterpreted as atypical pneumonia or unresolving pneumonia, with findings ranging from subclinical interstitial micronodular infiltrates to larger inflammatory pulmonary nodules.
 Treatment: typically aspirin and IV immunoglobulin.
Treatment: typically aspirin and IV immunoglobulin.
• Behçet disease
 Behçet disease is a relapsing multisystem disorder characterized by recurrent oral ulcerations and at least two of the following findings: genital ulcers, uveitis, cutaneous nodules or pustules, and positive pathergy test.8 A more recent scoring system provides better sensitivity for the diagnosis.9
Behçet disease is a relapsing multisystem disorder characterized by recurrent oral ulcerations and at least two of the following findings: genital ulcers, uveitis, cutaneous nodules or pustules, and positive pathergy test.8 A more recent scoring system provides better sensitivity for the diagnosis.9
 Vessels of all sizes in both the arterial and venous systems may be affected, although it is most common to have arterial small vessel or venous involvement.
Vessels of all sizes in both the arterial and venous systems may be affected, although it is most common to have arterial small vessel or venous involvement.
 While cough, dyspnea, or chest pain may represent initial respiratory symptoms, massive hemoptysis may be the most significant complication. Credited as the underlying mechanism in Behçet disease, immune complex deposition can lead to lung findings such as pulmonary artery aneurysms due to destruction of the elastic lamina or arterial–bronchial fistulae due to erosion of the bronchi.10 Resultant massive hemoptysis carries an associated mortality of nearly 40%. Pulmonary angiography has given way to CT and magnetic resonance angiography in the diagnosis of Behçet disease.
While cough, dyspnea, or chest pain may represent initial respiratory symptoms, massive hemoptysis may be the most significant complication. Credited as the underlying mechanism in Behçet disease, immune complex deposition can lead to lung findings such as pulmonary artery aneurysms due to destruction of the elastic lamina or arterial–bronchial fistulae due to erosion of the bronchi.10 Resultant massive hemoptysis carries an associated mortality of nearly 40%. Pulmonary angiography has given way to CT and magnetic resonance angiography in the diagnosis of Behçet disease.
 Treatment
Treatment
 Prednisone with azathioprine or cyclophosphamide produces the best outcomes for pulmonary artery aneurysms, although chlorambucil, colchicine, cyclosporine, and methotrexate in combination with prednisone have been used.
Prednisone with azathioprine or cyclophosphamide produces the best outcomes for pulmonary artery aneurysms, although chlorambucil, colchicine, cyclosporine, and methotrexate in combination with prednisone have been used.
 Aspirin at 81 mg/d should be considered for the prevention of recurrent venous thrombosis but should be avoided in any patient with known pulmonary involvement, given the risk of hemoptysis.
Aspirin at 81 mg/d should be considered for the prevention of recurrent venous thrombosis but should be avoided in any patient with known pulmonary involvement, given the risk of hemoptysis.
• Secondary vasculitides
 Both RA and SLE are associated with a secondary vasculitis thought to be immune complex mediated. Complications include rheumatoid nodules in the lungs in RA and pulmonary hypertension and alveolar hemorrhage in SLE. While the mortality associated with alveolar hemorrhage in SLE is considerable, treatment with the combination of plasmapheresis and pulse-dose cyclophosphamide has limited success.
Both RA and SLE are associated with a secondary vasculitis thought to be immune complex mediated. Complications include rheumatoid nodules in the lungs in RA and pulmonary hypertension and alveolar hemorrhage in SLE. While the mortality associated with alveolar hemorrhage in SLE is considerable, treatment with the combination of plasmapheresis and pulse-dose cyclophosphamide has limited success.
 Necrotizing sarcoid granulomatosis is distinguished from sarcoidosis by its extensive vasculitis and necrosis, lack of extrapulmonary involvement, and radiographic findings of pulmonary masses, nodules, and pleural involvement (all less commonly seen in sarcoidosis). The vasculitis may be epithelioid granulomatous (with histiocytes and multinucleated giant cells reminiscent of GCA) or lymphocytic without granuloma formation. Necrotizing sarcoid granulomatosis often features a subacute clinical onset and may include nonspecific respiratory symptoms such as cough, dyspnea, or wheezing. While prognosis is good (with spontaneous resolution seen in some cases), further therapy can include oral corticosteroids (similar to chronic pulmonary sarcoidosis).
Necrotizing sarcoid granulomatosis is distinguished from sarcoidosis by its extensive vasculitis and necrosis, lack of extrapulmonary involvement, and radiographic findings of pulmonary masses, nodules, and pleural involvement (all less commonly seen in sarcoidosis). The vasculitis may be epithelioid granulomatous (with histiocytes and multinucleated giant cells reminiscent of GCA) or lymphocytic without granuloma formation. Necrotizing sarcoid granulomatosis often features a subacute clinical onset and may include nonspecific respiratory symptoms such as cough, dyspnea, or wheezing. While prognosis is good (with spontaneous resolution seen in some cases), further therapy can include oral corticosteroids (similar to chronic pulmonary sarcoidosis).
Granulomatosis with Polyangiitis
GENERAL PRINCIPLES
• GPA, formerly known as Wegener granulomatosis, is a multisystem disease primarily involving small- and occasionally medium-sized blood vessels. While GPA was originally described as a variant of PAN, the findings of a progressive granulomatous process that involved the upper and lower respiratory tract led the German pathologist Frederick Wegener to believe that he had discovered a unique vasculitic syndrome. Recently, concerns have been raised about Wegener’s association with the Nazi regime. In 2011, the American College of Rheumatology (ACR), the American Society of Nephrology, and the European League Against Rheumatism formally changed the name to GPA.11
• The Chapel Hill Consensus Conference defined GPA as “granulomatous inflammation involving the respiratory tract, and necrotizing vasculitis affecting small- to medium-sized vessels.”1
• In terms of classification, GPA can be divided into limited and generalized disease.
 Limited GPA includes cases without kidney involvement (generally, limited to the upper respiratory tract or the lungs) and reflects pathology mainly due to necrotizing granulomas and not active vasculitis.
Limited GPA includes cases without kidney involvement (generally, limited to the upper respiratory tract or the lungs) and reflects pathology mainly due to necrotizing granulomas and not active vasculitis.
 Generalized GPA features pathology characterized by vasculitis and/or with any evidence of end-organ disease or impending organ failure.
Generalized GPA features pathology characterized by vasculitis and/or with any evidence of end-organ disease or impending organ failure.
• While GPA can occur at any age, ANCA-associated vasculitis typically affects middle-aged and older adults. GPA affects men and women equally but has a predilection for Caucasians.
DIAGNOSIS
Clinical Presentation
• The initial presentation of GPA may be insidious, with generalized complaints such as malaise, fatigue, weight loss, hearing loss, and upper respiratory symptoms. Soon after, patients may develop symptoms that involve multiple organ systems. Limited GPA tends to feature constitutional symptoms (and may progress to generalized GPA if left untreated), while generalized GPA can involve end-organ disease.
 Ear, nose, and throat involvement is present in up to 99% of cases of GPA, and may include chronic rhinitis and/or sinusitis, sinus pain, epistaxis, and nasal crusting. Destruction of the nasal cartilage can lead to nasal septal perforation or the saddle-nose deformity. Other manifestations include ulcerations of the oropharynx, gingival hyperplasia, and the rare strawberry gingival hyperplasia pathognomonic of GPA.12
Ear, nose, and throat involvement is present in up to 99% of cases of GPA, and may include chronic rhinitis and/or sinusitis, sinus pain, epistaxis, and nasal crusting. Destruction of the nasal cartilage can lead to nasal septal perforation or the saddle-nose deformity. Other manifestations include ulcerations of the oropharynx, gingival hyperplasia, and the rare strawberry gingival hyperplasia pathognomonic of GPA.12
 Patients can also develop symptoms that can be confused with asthma from tracheobronchial ulcerations, intraluminal inflammatory pseudotumor, and bronchomalacia. Scarring from these lesions can lead to significant airway obstruction.
Patients can also develop symptoms that can be confused with asthma from tracheobronchial ulcerations, intraluminal inflammatory pseudotumor, and bronchomalacia. Scarring from these lesions can lead to significant airway obstruction.
 The primary pulmonary manifestations of GPA include necrotizing granulomas, cavitary lesions, and scattered nodules. Capillaritis in the lung can lead to diffuse alveolar hemorrhage with an associated mortality of nearly 50%. This clinical presentation may be indistinguishable from Goodpasture syndrome or MPA.
The primary pulmonary manifestations of GPA include necrotizing granulomas, cavitary lesions, and scattered nodules. Capillaritis in the lung can lead to diffuse alveolar hemorrhage with an associated mortality of nearly 50%. This clinical presentation may be indistinguishable from Goodpasture syndrome or MPA.
 Dermatologic findings in GPA span papules, vesicles, palpable purpura, ulcers, or SC nodules. Leukocytoclastic vasculitis represents the most common manifestation, present in almost one-half of cases of GPA. Other skin lesions such as pyoderma gangrenosum and granulomatous skin lesions have been reported.
Dermatologic findings in GPA span papules, vesicles, palpable purpura, ulcers, or SC nodules. Leukocytoclastic vasculitis represents the most common manifestation, present in almost one-half of cases of GPA. Other skin lesions such as pyoderma gangrenosum and granulomatous skin lesions have been reported.
 Nervous system involvement is thought to be secondary to vasculitis of the vasa nervorum. Most commonly, patients may have mononeuritis multiplex, typically a sensorimotor polyneuropathy with asymmetric involvement (i.e., foot or wrist drop). Less commonly, patients may have cranial neuritis, cerebral vasculitis, or granulomatous infiltration.
Nervous system involvement is thought to be secondary to vasculitis of the vasa nervorum. Most commonly, patients may have mononeuritis multiplex, typically a sensorimotor polyneuropathy with asymmetric involvement (i.e., foot or wrist drop). Less commonly, patients may have cranial neuritis, cerebral vasculitis, or granulomatous infiltration.
 Renal involvement in GPA due to capillaritis leads to a pauci-immune crescentic glomerulonephritis. If left untreated, the renal disease may lead to end-stage renal failure.
Renal involvement in GPA due to capillaritis leads to a pauci-immune crescentic glomerulonephritis. If left untreated, the renal disease may lead to end-stage renal failure.
TABLE 20-2 AMERICAN COLLEGE OF RHEUMATOLOGY 1990 CRITERIA FOR DIAGNOSIS OF GRANULOMATOSIS WITH POLYANGIITIS

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


