After reading this chapter you will be able to: Venous thromboembolism is a major health problem in the United States. The prevalence of venous thromboembolism, which includes PE and DVT, has remained relatively constant over time and has been calculated to be 117 cases per 100,000 persons (i.e., DVT at 48 cases per 100,000 and PE at 69 cases per 100,000). It is estimated that 200,000 to 300,000 new cases occur yearly in the United States.1 Venous thromboembolism is treatable but requires prompt diagnosis and therapy to avert serious consequences. One-third of deaths from PE occur within 1 hour of onset of symptoms, and more than 70% of patients who die of PE are not suspected to have PE before death.2 Although mortality from PE has decreased in recent years,3 the death rate for the first episode of PE among hospitalized patients may be 17.4% at 3 months.3,4 Recurrent PE is associated with a much higher mortality rate because only one-quarter of patients survive 3 months.5 The diagnosis is not suspected in approximately two-thirds of patients who die of PE, and the frequency of recognizable emboli in routine autopsies of adult patients ranges from 1.5% to almost 30%.6–8 In a population-based study of PE as a cause of death in New Mexico, only 34% of 812 postmortem documented cases of PE were diagnosed before death.5 Morpurgo and Schmid9 reported their experience with 92 postmortem cases of massive or submassive PE detected during the years 1986-1989. Only 28% of the cases were diagnosed before death, a finding that emphasizes the underdiagnosis of venous thromboembolic disease. Two-thirds of cases of initial embolus from which patients survive remain undiagnosed. The mortality rate among these patients with undiagnosed embolism is approximately 30%,10 which emphasizes the importance of recognizing PE. If venous thromboembolism is recognized and treated, the mortality rate decreases to less than 8%, and the long-term outcome is generally favorable.11 The long-term survival rates after venous thromboembolism in an inception cohort of 2218 patients were 72% at 1 month and 63% at 1 year.12 Because the accuracy of the clinical impression (i.e., without testing) of venous thromboembolism is less than 50%,13 objective tests are needed to confirm or exclude the diagnosis. Patients with multiple injuries, immobilization, bed rest, or intravascular catheters and elderly patients are at high risk of venous thromboembolic disease (Table 26-1) and should be considered candidates for testing when appropriate symptoms develop. TABLE 26-1 Frequency of Venous Thrombosis in Various Hospitalized Patient Groups From Arroliga AC, Matthay MA, Matthay RA: Pulmonary thromboembolism and other pulmonary vascular diseases. In George RB, et al, editors: Chest medicine: essentials of pulmonary and critical care medicine, ed 3, Baltimore, 1995, Williams & Wilkins. Pulmonary emboli arise from detached portions of venous thrombi that form, in most cases, in deep veins of the lower extremities or pelvis (86%); the point of origin of pulmonary emboli is actually found in only one-half of patients.9 A small percentage of pulmonary emboli arise from the right-sided heart chambers (3.15%) or the superior vena cava (3%).9 Conditions that favor thrombus formation include blood stasis, the presence of hypercoagulable states, and vessel wall abnormalities (factors known as Virchow’s triad). Causes of blood stasis include local pressure, venous obstruction, and immobilization. Other causes of stasis include congestive heart failure, shock and dehydration, varicose veins, and enlargement of the right heart chambers. Several conditions enhance the intravascular coagulability of the blood and predispose to venous thromboembolic disease (Box 26-1).14 The most frequent causes of an inherited hypercoagulable state are the factor V Leiden mutation and the prothrombin gene mutation, which together account for 50% to 60% of cases.15 The major acquired risk factors for venous thromboembolism include recent major surgery, trauma, immobilization, antiphospholipid antibody syndrome, malignancy, pregnancy, oral contraceptives, and myeloproliferative disorders.16 Vessel wall abnormalities are found most often in patients who have sustained trauma or have undergone major surgery. Stasis, an important factor for the formation of DVT, is rarely the only risk factor.17 Deposition of platelets and fibrin in the venous valve cups of the lower extremities occurs as a result of stasis. The combination of diminished blood flow and the presence of trauma and toxins can worsen endothelial damage and promote the release of mediators that encourage adhesion, aggregation, and degranulation of platelets. The result is activation of the coagulation cascade and production of thrombi and fibrin. PE is a frequent complication of DVT, occurring in more than 50% of cases with DVT confirmed on phlebography.18 PE occurs when a fragment of the thrombus in the venous system travels to the pulmonary circulation. Pulmonary emboli occur more frequently in the lower lobes and are more often found in the right lung than in the left lung, a phenomenon probably related to the flow distribution that favors the right lung and the lower lobes.6 Embolism to the pulmonary circulation produces pulmonary hemorrhage in the ischemic or infarcted lung in less than 10% of cases of PE. Infarction, secondary to thromboembolism, is less common in the lung than in other tissues because the lung has two blood supplies: the pulmonary arterial circulation and the bronchial circulation. At a capillary level, extensive connections exist within the pulmonary and bronchial circulations that prevent serious damage to lung tissue deprived of its pulmonary artery supply.6 Patients with underlying cardiovascular disease may have impairment of the remaining bronchial circulation with resultant lung tissue necrosis when emboli occur. Pulmonary infarction is associated with thromboembolic obstruction of a medium-sized pulmonary artery. Generally, infarcts occur at the lung bases, are pleural-based, and may be accompanied by pleural effusion. Microscopic examination of the lung in pulmonary infarction shows necrosis of alveolar walls, alveoli filled with red blood cells, and a mild inflammatory response in the periphery.6 The sudden obstruction of a pulmonary arterial branch causes a decrease in or total cessation of blood flow to the distal area of the lung that leads to respiratory and hemodynamic alterations.19 In the appropriate context, massive PE should be suspected anytime there is unexplained hypotension accompanied by an elevated central venous pressure (jugular vein distention).20 It is a catastrophic entity that frequently results in acute right ventricular failure and death. Death from massive PE is the result of cardiovascular collapse rather than of respiratory failure. Embolic obstruction of the pulmonary artery increases the alveolar dead space, causes bronchoconstriction, and decreases the production of alveolar surfactant. Wasted or dead space areas occur when areas of the lung parenchyma are ventilated but not perfused. The response is to increase total ventilation ( Not all patients with PE have significant arterial hypoxemia, but the presence of a widened alveolar-arterial oxygen (O2) tension gradient and reduced PaO2 are common. Hypoxemia develops because of The main hemodynamic consequence of PE is increased resistance to blood flow caused by obstruction of the pulmonary arterial bed. The hemodynamic consequences are determined by the extent of the cross-sectional area of the pulmonary circulation involved, the underlying cardiopulmonary reserve, and the neurohumoral response to the embolism. Pulmonary hypertension occurs when 50% of the pulmonary vascular bed has been occluded.19,21 To maintain the same flow at a higher pressure, the right ventricle must work harder. The result is an increase in right ventricular work that causes the right ventricle to become dilated and ischemic. The thin-walled right ventricle is not designed to work with acute heavy pressure loads. When the mean pulmonary artery pressure increases to greater than 40 mm Hg during an acute first PE, the right ventricle fails, and hemodynamic collapse and death occur.22 The exact role of vasoconstriction in the pathogenesis of pulmonary hypertension is uncertain, but vasoconstrictors such as serotonin and thromboxane A2 may also play a role in the development of pulmonary hypertension after acute PE. Although the usual course of PE is to resolve rapidly (because the body lyses the embolism with endogenous fibrinolytic agents), permanent residual emboli do occur.23 Massive emboli are likely to resolve within weeks, particularly in young patients. Overall, less than 10% of patients have perfusion defects after 6 weeks. Vascular patency is restored when the unresolved emboli organize or form scars against the vessel wall. A high index of suspicion for venous thromboembolism is crucial to make the diagnosis for patients at risk. No specific signs or symptoms indicate the presence of venous thromboembolic disease, and a significant proportion of patients are asymptomatic (32%).2,24 The physical findings of DVT in the lower extremities include erythema and warm skin in one-third of patients and swelling and tenderness in three-fourths of patients. In patients who have swelling above and below the knee, fever, and a history of immobility and cancer, the likelihood of finding DVT on a venogram is only 42%.25 The most frequent symptoms in patients with confirmed PE are dyspnea, followed by pleuritic chest pain and cough (Table 26-2).26 The onset of dyspnea is usually rapid, within seconds (46%) or minutes (26%).26 Hemoptysis occurs in 13% to 20% of patients. The combination of dyspnea of sudden onset, fainting, and acute chest pain should raise suspicion of PE. In one study, this combination of symptoms was present in 96% of patients with confirmed PE compared with 59% of patients in whom PE was suspected but not confirmed.27 In some patients, dyspnea lasts only a few minutes, and this episode may be wrongly dismissed as being trivial.19,27–29 TABLE 26-2 Clinical Characteristics in Patients With Pulmonary Embolism and No Cardiopulmonary Disease Modified from Stein PD, Beemath A, Matta F, et al: Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 120:871–879, 2007. There are no characteristic physical findings of PE. The most frequent physical findings include tachypnea, rales on chest examination, and tachycardia. Similar to dyspnea, these signs may be short-lived. Other common physical findings include an accentuated pulmonary component of the second heart sound (loud P2) consistent with pulmonary hypertension. Fever may be present in 54% of patients.27–29 Similar to what occurs in the diagnosis of DVT, less than 35% of patients in whom PE is clinically suspected actually have it.2 The chest radiograph cannot confirm the presence of PE but is helpful to rule out other potentially life-threatening conditions, such as pneumothorax or pneumonia, which can manifest in a similar way. In dyspneic patients, a normal chest radiograph may be a clue to the presence of PE; however, chest radiography is abnormal in more than 80% of cases. Abnormalities include enlargement of the right descending pulmonary artery (66%), elevation of the diaphragm (61%), cardiomegaly (55%), and small pleural effusion (50%). Parenchymal densities (patchy infiltrates or round nodular lesions) predominantly appearing next to the pleural surface are present in patients who have infarction or atelectasis. Other, less common findings include the Westermark sign, in which there is pulmonary hyperlucency caused by a marked reduction in blood flow. The so-called Hampton hump, an opacity in the costophrenic angle, is present in 25% to 30% of patients.27 The electrocardiogram (ECG) is helpful to rule out other diagnoses, such as acute myocardial infarction and pericarditis. The ECG is frequently abnormal in patients with PE (87% of the time), but the ECG abnormalities accompanying PE are nonspecific in 70% to 75% of cases; tachycardia and ST segment depression are most common.27 Abnormalities such as T wave inversion in right precordial leads, depression of the ST segment, and T wave inversion in V1 and V2 may be present. A so-called S1Q3T3 pattern is associated with massive PE and is present in 19% of such patients.25 Most patients with acute PE have hypoxemia and hypocapnia,27 but a significant percentage of patients (15% to 25%) with or without previous cardiopulmonary disease have a PaO2 exceeding 80 mm Hg.29 Although a widened alveolar-arterial O2 gradient is frequently present, a normal alveolar-arterial O2 gradient may occur in approximately 20% of patients with angiographically documented PE.27,29 Although an arterial blood gas measurement may be helpful in identifying patients with hypoxemia or hypocapnia accompanying PE, arterial blood gas measurements can never secure the diagnosis of PE. Clot formation is invariably associated with thrombingeneration. Measurement of cross-linked fibrin split products (D-dimers) has been found to be sensitive for the diagnosis of acute venous thromboembolism. The specificity of D-dimer enzyme-linked immunosorbent assay (ELISA) can exclude all but 5% to 10% of patients with acute PE, so this test has been used as an important tool for early assessment.30 The specificity of the test is only 39%, but a value less than the recommended cutoff for current quantitative ELISA assays has been shown to rule out venous thromboembolic disease in 98% of patients.31–33 In patients in whom DVT is suspected clinically, negative results of the D-dimer assay combined with negative findings on impedance plethysmography have a negative predictive value of 98% for DVT (i.e., if the test is negative, the chance of a DVT is only 2%).31 D-dimer results have been particularly useful in the emergency department and outpatient area for the evaluation of patients with suspected DVT31 and PE.33 In patients with a low pretest probability of DVT or PE and a negative D-dimer result, the negative predictive value for the strategy has been greater than 99%.32,33 Although there are several laboratory methods to measure D-dimer levels, tests using ELISAs are the most widely used and best performing among the D-dimer assays regarding the sensitivity and negative likelihood ratio. For excluding PE or DVT, a negative result on quantitative rapid ELISA is as diagnostically useful as a normal lung scan or negative duplex ultrasonography finding. D-dimer ELISA can be used to exclude PE in outpatients with a low to moderate suspicion without the need for further costly testing. However, inpatients should undergo an imaging study as the initial test for PE because most will already have elevated D-dimer levels because of comorbid conditions.33 To evaluate the clinical pretest probability of DVT, the Wells criteria are frequently used. These criteria include the following clinical parameters: presence of cancer, immobilization, localized tenderness, swelling, edema, previous DVT, collateral superficial veins, and absence of an alternative diagnosis.34 In cases in which there is a moderate to high pretest probability, several modalities could be used for diagnosing DVT in the extremities, such as compression ultrasonography, impedance plethysmography, and venography. In patients with low pretest probability of DVT and a negative D-dimer, further testing may be unnecessary.31,35–37 Impedance plethysmography is a noninvasive method and measures electrical impedance to blood flow, which changes with inflation and deflation of a lower extremity cuff. The quality of the test is operator-dependent and requires that the patient be supine and lay still for at least 2 minutes. The test has a sensitivity and specificity of 91% and 96% for symptomatic proximal venous thrombi. A lower sensitivity of 65% has been reported.38,39 Compression ultrasonography has proved to be sensitive and specific for the diagnosis of symptomatic proximal DVT. This test is noninvasive, portable, and accurate and is the modality of choice for the diagnosis of DVT. Compression ultrasonography combines B-mode scanning with a tightly focused pulse Doppler beam directed at the vessels of interest. DVT is diagnosed with the findings of venous noncompressibility, an echogenic filling defect, absence of Doppler flow, free-floating thrombus in the vein, and venous distention.40 The most reliable sign of DVT is lack of compressibility of the vein, although a free-floating thrombus has the highest embolic potential (Figure 26-1). The sensitivity and specificity of compression ultrasonography in symptomatic patients vary between 95% and 100% for the detection of a proximal lower extremity thrombus.40,41 Areas not well visualized with compression ultrasonography include the iliac veins, the superficial femoral veins in the adductor canal, and the calf veins. However, the accuracy of compression ultrasonography, even with the addition of color Doppler ultrasonography, is moderate to low for the detection of DVT in patients at high risk who do not have symptoms.42 These results suggest that ultrasonography, although sensitive and specific for the diagnosis of DVT, is not a good screening test for patients at high risk who do not have symptoms. Noninvasive tests for the diagnosis of PE include TABLE 26-3
Pulmonary Vascular Disease
 State how many patients develop venous thromboembolism each year.
State how many patients develop venous thromboembolism each year.
 Describe how and where thromboemboli originate.
Describe how and where thromboemboli originate.
 Describe how pulmonary emboli alter lung and cardiac function.
Describe how pulmonary emboli alter lung and cardiac function.
 Identify the clinical features and electrocardiographic, chest radiograph, and arterial blood gas findings associated with pulmonary embolism.
Identify the clinical features and electrocardiographic, chest radiograph, and arterial blood gas findings associated with pulmonary embolism.
 Describe how pulmonary embolism is diagnosed and managed.
Describe how pulmonary embolism is diagnosed and managed.
 Describe hemodynamic findings associated with pulmonary hypertension.
Describe hemodynamic findings associated with pulmonary hypertension.
 Describe the possible mechanisms believed to be responsible for the onset of idiopathic pulmonary arterial hypertension (IPAH).
Describe the possible mechanisms believed to be responsible for the onset of idiopathic pulmonary arterial hypertension (IPAH).
 State patients who are at risk for the development of IPAH.
State patients who are at risk for the development of IPAH.
 Identify the clinical features associated with IPAH.
Identify the clinical features associated with IPAH.
 Describe the treatment for patients with IPAH.
Describe the treatment for patients with IPAH.
 Describe the pathogenesis and management of pulmonary hypertension associated with chronic obstructive pulmonary disease.
Describe the pathogenesis and management of pulmonary hypertension associated with chronic obstructive pulmonary disease.
Venous Thromboembolic Disease
Group
Frequency (%)
Orthopedic (e.g., fractured hip)
54-67
Urologic (e.g., prostatectomy)
25
Surgical patients >40 yr old
28
Gynecologic surgery
18
Cardiovascular surgery (e.g., acute myocardial infarction)
39
Obstetrics
3
Pathogenesis
Pathology
Pathophysiology
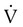 ). The increased
). The increased 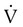 contributes to the sensation of dyspnea that accompanies PE. Bronchoconstriction from diminished carbon dioxide concentration, regional hypoxia, and the production of serotonin and histamine cause further ventilation/perfusion (
contributes to the sensation of dyspnea that accompanies PE. Bronchoconstriction from diminished carbon dioxide concentration, regional hypoxia, and the production of serotonin and histamine cause further ventilation/perfusion (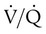 ) mismatching.21
) mismatching.21
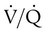 mismatch, intrapulmonary shunt, and cardiogenic shock. Shock is caused by obstruction of the pulmonary vasculature by massive emboli or by numerous small emboli in the presence of cardiopulmonary disease. Cardiac output decreases, and O2 delivery declines. With the decrease in O2 delivery, the peripheral tissues increase O2 extraction causing venous O2 desaturation. In patients with significantly increased right heart pressures, intracardiac right-to-left shunt may develop when blood flows through a patent foramen ovale.19,21 In addition, the depletion of surfactant material as a result of embolic occlusion can lead to atelectasis and intrapulmonary shunt, which can cause hypoxemia.19
mismatch, intrapulmonary shunt, and cardiogenic shock. Shock is caused by obstruction of the pulmonary vasculature by massive emboli or by numerous small emboli in the presence of cardiopulmonary disease. Cardiac output decreases, and O2 delivery declines. With the decrease in O2 delivery, the peripheral tissues increase O2 extraction causing venous O2 desaturation. In patients with significantly increased right heart pressures, intracardiac right-to-left shunt may develop when blood flows through a patent foramen ovale.19,21 In addition, the depletion of surfactant material as a result of embolic occlusion can lead to atelectasis and intrapulmonary shunt, which can cause hypoxemia.19
Clinical Features
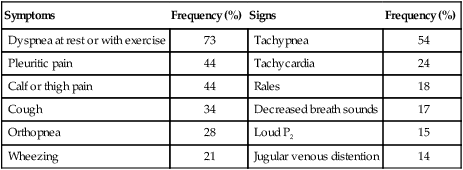
Symptoms
Frequency (%)
Signs
Frequency (%)
Dyspnea at rest or with exercise
73
Tachypnea
54
Pleuritic pain
44
Tachycardia
24
Calf or thigh pain
44
Rales
18
Cough
34
Decreased breath sounds
17
Orthopnea
28
Loud P2
15
Wheezing
21
Jugular venous distention
14
Chest Radiograph
Electrocardiogram
Arterial Blood Gases
Diagnostic Modalities
By-Products of Thrombin and Plasmin
Testing for Lower Extremity Deep Venous Thrombosis
Impedance Plethysmography
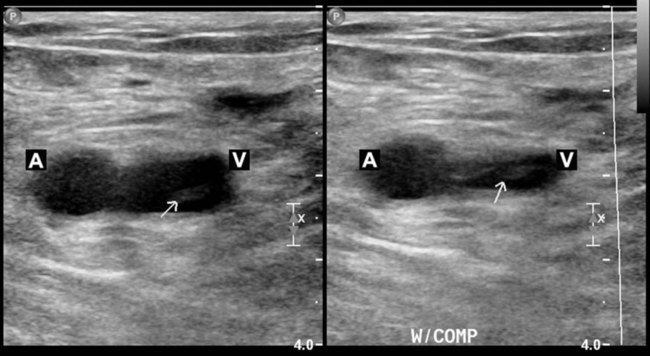
Compression Ultrasonography
Testing for Pulmonary Embolism
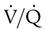 scan and helical or spiral computed tomography (CT) angiography scan of the chest. Either of these tests, depending on the resources available, may be the initial diagnostic examination if the presence of acute PE is clinically suspected.43 Echocardiography can suggest the diagnosis (right ventricular dilation, dysfunction, or thrombus) and provide prognostic information.44 In certain cases when these noninvasive tests are nondiagnostic, pulmonary angiography may be needed to confirm or exclude the diagnosis of PE.
scan and helical or spiral computed tomography (CT) angiography scan of the chest. Either of these tests, depending on the resources available, may be the initial diagnostic examination if the presence of acute PE is clinically suspected.43 Echocardiography can suggest the diagnosis (right ventricular dilation, dysfunction, or thrombus) and provide prognostic information.44 In certain cases when these noninvasive tests are nondiagnostic, pulmonary angiography may be needed to confirm or exclude the diagnosis of PE.
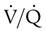 scanning involves the inhalation of a radiolabeled gas (usually xenon-133, xenon-127, krypton-181m, or technetium-99m) and the intravenous injection of macroaggregated albumin tagged with a gamma-emitting radioisotope. The distribution of lung ventilation (
scanning involves the inhalation of a radiolabeled gas (usually xenon-133, xenon-127, krypton-181m, or technetium-99m) and the intravenous injection of macroaggregated albumin tagged with a gamma-emitting radioisotope. The distribution of lung ventilation (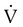 ) and lung perfusion (
) and lung perfusion (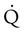 ) is studied, and areas of mismatch where
) is studied, and areas of mismatch where 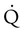 is less than
is less than 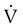 are sought. The presence of mismatches most often indicates embolic occlusion of the blood vessel, although other rare causes of mismatches exist, such as extrinsic compression of the vessel by a mass, intraluminal obstruction by angiosarcoma, or obliteration of a vessel by vasculitis. The addition of
are sought. The presence of mismatches most often indicates embolic occlusion of the blood vessel, although other rare causes of mismatches exist, such as extrinsic compression of the vessel by a mass, intraluminal obstruction by angiosarcoma, or obliteration of a vessel by vasculitis. The addition of 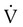 scan increases the specificity of
scan increases the specificity of 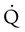 scan.45 Generally, with the presence of a parenchymal abnormality, the
scan.45 Generally, with the presence of a parenchymal abnormality, the 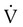 defect coincides with the
defect coincides with the 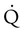 defect, and matched abnormalities are found. Normal results of a
defect, and matched abnormalities are found. Normal results of a 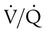 scan exclude the presence of a clinically significant PE in the context of a low clinical probability of PE. In these cases, anticoagulant therapy can be safely withheld.46 Abnormal
scan exclude the presence of a clinically significant PE in the context of a low clinical probability of PE. In these cases, anticoagulant therapy can be safely withheld.46 Abnormal 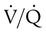 scan results can be classified as high probability, intermediate (or indeterminate) probability, and low probability for PE, according to the size of the defect and the degree of mismatch between the
scan results can be classified as high probability, intermediate (or indeterminate) probability, and low probability for PE, according to the size of the defect and the degree of mismatch between the 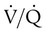 scan and chest radiographic abnormalities.45 Diagnostic accuracy is greatest when
scan and chest radiographic abnormalities.45 Diagnostic accuracy is greatest when 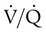 scan results are combined with clinical probability (Table 26-3).47 The presence of concomitant cardiopulmonary disease (e.g., COPD), even if severe, does not diminish the diagnostic usefulness of
scan results are combined with clinical probability (Table 26-3).47 The presence of concomitant cardiopulmonary disease (e.g., COPD), even if severe, does not diminish the diagnostic usefulness of 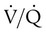 scans in the diagnosis of acute PE.48–50
scans in the diagnosis of acute PE.48–50
High probability
Two or more large (>75% of a segment) segmental 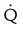 defects without corresponding
defects without corresponding 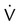 or abnormalities on chest radiograph
or abnormalities on chest radiograph
One large segment 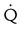 defect and two or more moderate (25%-75% of a segment) segmental
defect and two or more moderate (25%-75% of a segment) segmental 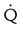 defects without corresponding
defects without corresponding 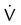 or abnormalities on chest radiograph
or abnormalities on chest radiograph
Four or more moderate segmental 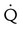 defects without corresponding
defects without corresponding 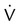 or abnormalities on chest radiograph
or abnormalities on chest radiograph
Intermediate probability
One moderate or up to two large segment 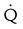 defects without corresponding
defects without corresponding 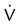 defect or abnormalities on chest radiograph
defect or abnormalities on chest radiograph
Corresponding 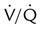 defects and parenchymal opacity in lower lung zone on chest radiograph
defects and parenchymal opacity in lower lung zone on chest radiograph
Corresponding 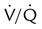 defects and small pleural effusion
defects and small pleural effusion
Single moderate matched 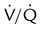 defects with normal findings on chest radiograph
defects with normal findings on chest radiograph
Findings difficult to categorize as normal, low, or high probability
Low probability
Multiple matched 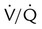 defects, regardless of size, with normal findings on chest radiograph
defects, regardless of size, with normal findings on chest radiograph![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access