Pulmonary Thromboembolic Disease
INTRODUCTION
Pulmonary thromboembolic disease refers to the condition in which blood clot(s) (thrombus or multiple thrombi) migrate from the systemic circulation to the pulmonary vasculature. Most of these thrombi arise from the deep veins of the lower and upper extremities (deep venous thrombosis [DVT]). From the clinical standpoint, DVT and pulmonary embolism (PE) can be considered a continuum of the same disease, and the two terms are often collectively referred to as venous thromboembolism.
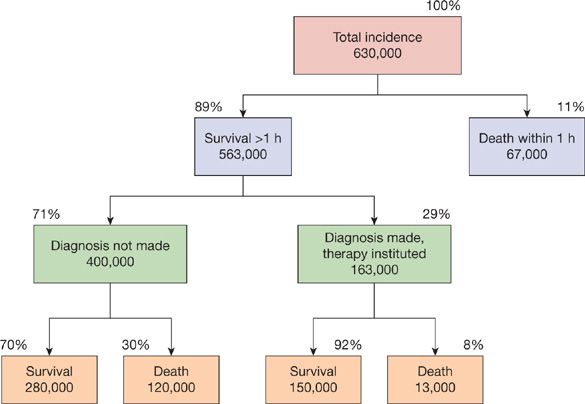
The annual incidence of PE in the United States remains uncertain. In a retrospective analysis of data involving 2218 Olmsted County residents over a 10-year period, community residents who were not hospitalized within a 90 days period had an incidence of PE of 3.6 per 10,000 person-years.1 A slightly lower incidence of 2.3 per 10,000 person-years was reported in an earlier study in Massachusetts.2 This translates to an annual incidence of approximately 100,000 cases in the United States. However, the true incidence of PE is likely to be much higher since many cases remain undiagnosed. A recent systematic review revealed that silent PE was present in 32% of patients with DVT.3 An earlier report estimated that as many as 630,000 patients develop PE every year in the United States with 200,000 related deaths, the majority in patients in whom the diagnosis was never made (Fig. 73-1).4 Although considerable effort is directed toward the development of new diagnostic techniques and therapeutic agents, a considerable impact on mortality related to the disease would arise from the routine use of prophylactic strategies, an understanding of the often subtle clinical presentation of the disease, and the appropriate application of existing diagnostic techniques.
Figure 73-1 Estimated incidence and survival statistics for pulmonary embolism in the United States. (Reproduced with permission from Dalen JE, Alpert JS. Natural history of pulmonary embolism. Prog Cardiovasc Dis. 1975;17(4):259–270.)
SOURCES OF EMBOLI
Most cases (80%–95%) of PE occur as a result of thrombus originating in the lower extremity. Thrombus often begins at a site where blood flow is turbulent, such as at a venous bifurcation, or behind a venous valve (Fig. 73-2). When thrombus propagation exceeds the rate of thrombus organization and adherence to the endothelium, part or all of thrombus may break away and migrate via the venous system to the lungs. Most thrombi originate in the deep veins of the calf and propagate proximally to the popliteal and femoral veins. Calf-limited thrombi pose a minimal embolic risk while those that extend into and above the popliteal vein represent the most common source of acute symptomatic PE. This is not meant to imply that calf-limited thrombosis represents a benign condition. Proximal propagation may occur in as many as 15% of untreated patients along with a higher risk of thrombotic recurrence and postphlebitic syndrome.5
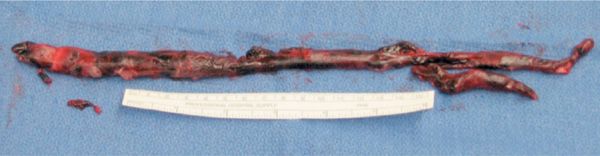
Figure 73-2 Large, well-organized embolus representing “cast” of a lower extremity vein removed from pulmonary artery at pulmonary embolectomy.
Emboli may originate from other sources, most often from the pelvic veins, in which case a predisposing factor such as pregnancy, pelvic thrombophlebitis or pelvic infection, prostate disease, or recent pelvic surgery can often be identified. Emboli may also originate from upper extremity thrombosis associated with central venous catheters or intravascular cardiac devices, or may be associated with thoracic outlet obstruction or effort thrombosis (Paget–von Schroetter syndrome).6 A small number of patients with PE may have evidence of right ventricular thrombus at presentation and this has been associated with more hemodynamic instability and an increase in mortality.7
Although the majority of cases of PE are the result of thrombus migration (hence, thromboembolism), other materials may occasionally obstruct the pulmonary vascular bed. These include blood-borne parasites (such as schistosomiasis), sickle cell disease, and various “contaminants” of illicit injected drugs (talc, cloth fibers, etc.). Air embolism is usually iatrogenic and typically enters the blood stream accidentally through a central venous catheter. Less commonly, a patient’s own tissues or cells may enter the blood stream and lodge in the pulmonary vasculature. Examples include amniotic fluid embolism, which can occur during or immediately after labor or late-term abortion; fat embolism, which is usually associated with long bone fractures; and tumor embolism. PE due to sickle cell disease is caused by “clumping” of abnormal red blood cells in the setting of hypoxia and stress, and can cause both acute respiratory distress as well as a more progressive disease with secondary pulmonary hypertension.
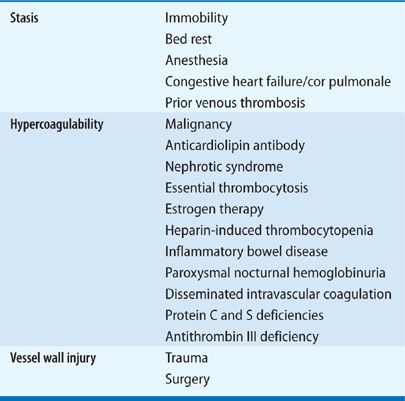
PREDISPOSING FACTORS
Rudolf Virchow first described the phenomena of “embolism” and “thrombosis” in the mid-nineteenth century, and identified three main factors contributing to the formation of venous thrombosis (Virchow’s triad): venous stasis, hypercoagulability, and injury to the venous wall (endothelium). One hundred and fifty years later, this basic classification remains useful in helping clinicians stratify individual patient’s risk of developing venous thromboembolism (Table 73-1). It is important to recognize that there is an interplay between acquired and genetic risk factors, that multiple mechanisms lead to the development of DVT and/or PE, and that multiple factors can often be found in individual patients.
 ACQUIRED RISK FACTORS
ACQUIRED RISK FACTORS
The risk imposed by a major surgical procedure is well recognized. Without prophylaxis, venous thrombosis occurs after approximately 20% of all major surgical procedures with associated embolism after 1% to 2%.8 The incidence of thromboembolism without prophylaxis is even higher in orthopedic patients with over 50% of major orthopedic procedures complicated by venous thrombosis.9 In the absence of prophylaxis, the frequency of fatal postoperative PE ranges from 0.1% to 0.4% in patients undergoing elective general surgery and from 1% to 5% in patients undergoing elective hip or knee surgery, emergency hip surgery, major trauma, or spinal cord injury.
Major traumatic injuries, most notably those of the head, spine, and pelvis, are also associated with high risk. The basis for this risk is multifactorial, involving all three components of Virchow’s triad.
Although initially recognized and studied in surgical patients, it is now appreciated that hospitalized medical patients may be equally prone to develop DVT.10 In about 80% of the cases, one or more risk factors may be present when extensive investigative testing is performed. Major risk factors include New York Heart Association class III and IV congestive heart failure, chronic obstructive pulmonary disease, sepsis and other inflammatory disorders, advanced age, stroke, critical illness, and prolonged bed rest.
Any prolonged period of immobilization may increase thromboembolic risk and explains the occurrence of thrombosis under such circumstances as paralysis, bed rest, and prolonged air travel. Long distance traveling (economy class syndrome) is associated with a 1.5- to 3-fold increase in thromboembolic risk, depending on the travel duration.11 Despite the relative increase in risk, the actual incidence of PE associated with air travel is quite low.12
Pregnancy is the most common cause of venous thromboembolism in women younger than 40 years old, and if untreated may account for 20% to 50% of all pregnancy-related deaths. Compared with nonpregnant women, the risk of venous thrombotic events is increased fivefold during pregnancy and 60-fold in the first 3 months after delivery.13,14 The increase may be a result of decreased mobility, pregnancy-related hypercoagulable state (increases in factors II, VII, VIII, X, acquired activated protein C resistance, and decreased free protein S level), and venous obstruction from uterine compression. The incidence is estimated at 0.76 to 1.72 cases per 1000 pregnancies and occurs in roughly equal distribution over all trimesters. Most cases of postpartum DVT occur within the first 6 weeks after delivery. Cesarean section, premature birth, multiple births, preeclampsia, advance maternal age, and maternal history of cardiac disease have all been identified as contributing factors. Interestingly, 90% of all DVT cases are noted in the left leg, presumably because of the anatomic relationship between the uterus and inferior vena cava (IVC).
The use of oral contraceptive agents and hormonal replacement therapy has also been associated with an increased risk of venous thromboembolism.14,15 Although the risk of venous thromboembolism is higher among users of oral estrogen-containing contraceptives, the absolute risk is low. In terms of oral contraceptive agents, the relative risk of developing venous thrombosis is a four- to sixfold increased risk. There appears to be a synergistic effect of oral contraceptives with obesity.16
Hormone replacement therapy appears to be associated with a two- to fourfold increased risk of venous thromboembolism.17 Studies have suggested that venous thromboembolic risk is lower with transdermal hormone preparations. However, none of these studies are randomized trials. Given that the baseline risk of thrombosis increases with age, the use of hormonal replacement therapy in a postmenopausal population has a considerably higher impact on absolute rates of thrombosis.
Obesity has been associated with venous thromboembolism, particularly in women. The Nurses’ Health Study found that a body mass index greater than or equal to 29 kg/m2 was an independent risk factor.18 The metabolic syndrome, defined by abdominal obesity, elevation of blood pressure, elevated fasting blood sugar and triglycerides, and low levels of high-density lipoprotein cholesterol, appears to be associated not only with an increased risk of atherosclerotic disease but also of venous thromboembolism.19
The risk of venous thromboembolism increases with age. A recent study, using hospital discharge surveys over a 21-year period, found that patients 70 years or older have an approximately 25-fold increased risk, compared with those 20 to 29 years of age.20 Presumably the difference may be due to decrease in mobility and increase in comorbidities in this population. Elderly patients also appear to have a higher mortality due to PE, and PE is suspected less commonly prior to death in the elderly patient.
Cancer patients, particularly those with primary malignancies from lung, pancreas, breast (mucin-secreting adenocarcinoma), prostate, stomach/colorectal, and genitourinary tracts are at a high risk for venous thromboembolism. Cancer is estimated to increase the risk of venous thromboembolism by four- to sixfold. Patients with cancer also have a higher risk of thromboembolic recurrence and those with venous thromboembolism have a higher overall mortality rate than cancer patients without thrombosis. Multiple factors are probably involved and include the development of abnormalities in the hemostatic system related to the malignancy itself, hemostatic alterations induced by chemotherapeutic agents, immobility, infectious complications, and the presence of chronic indwelling central venous catheters. Although most instances of cancer-associated venous thromboembolism occur after the diagnosis of the malignancy, approximately 5% to 10% of patients with “idiopathic” venous thrombosis have a malignancy diagnosed within the next 2 to 3 years. There is no evidence at this time to recommend an aggressive search for cancer in patients with idiopathic or unprovoked thrombosis. Recent data suggest that a limited approach (routine laboratory testing, chest radiograph, tumor markers, abdominal ultrasound) may have the capacity to identify approximately one-half of malignancies in patients who were negative on routine examination.21 More extensive screening utilizing chest and abdominal computed tomography (CT) appears to result in excessive false-positive results without an effect on outcome.22
Various hematologic conditions such as polycythemia vera, essential thrombocytosis, and acute leukemia may result in significant overproduction of different cell lines, which in turn may increase the risk of venous thromboembolism by increasing blood viscosity (hyperviscosity syndromes) and through the release of procoagulants.23 This type of thrombosis seems to occur more frequently in the hepatic or portal veins and may be the presenting symptoms of the underlying disorder.
Paroxysmal nocturnal hemoglobinuria is a rare condition associated with an incidence of venous thromboembolism of approximately 40%.24 Many cases involve nonlower extremity sites, particularly in the intra-abdominal vessels. The reason for thrombosis is not clear but may be related to a decrease in blood complement levels in these patients.
The presence of antiphospholipid antibodies, most notably the lupus anticoagulant, appears to be an independent risk factor for venous thromboembolism.25 Among patients with venous thrombosis, a lupus anticoagulant has been reported in 5% to 15% and this abnormality has been estimated to lead to a ninefold increased risk of thrombosis.
The frequency of venous thromboembolism in patients with nephrotic syndrome may be as high as 40%.26 There is a higher tendency for the thrombosis to present in unusual locations such as the cerebral sinus or as arterial thrombosis. Rarely, thrombosis may also be the presenting symptom of the nephrotic syndrome. The mechanism for venous thromboembolism in these patients is not clear but various factors such as functional or quantitative changes in coagulation factors, diminished fibrinolytic activity, platelet hyperreactivity, and increased blood viscosity have been proposed.
Patients with inflammatory bowel disease are at substantially increased risk of both venous and arterial thromboses.27,28 The exact pathogenetic mechanism remains unclear. The majority of thrombotic complications occur during an active phase of the disease and inflammatory mechanisms have been proposed.
 INHERITED RISK FACTORS
INHERITED RISK FACTORS
Many patients who develop venous thromboembolism are found to have an inherited risk factor due to either abnormal levels of or functional abnormalities in coagulation factors (inherited thrombophilia). The relative risk of thrombosis varies widely depending on the hemostatic defect. In general, this group of patients tends to be younger (less than 50 years) and has a tendency to develop recurrent venous thromboembolism.
The first known inherited thrombophilic trait was antithrombin III deficiency, originally described in 1965. Subsequently, a number of other genetic mutations associated with venous thromboembolism have been reported. The most common of these inherited predispositions was first described in 1993 by Dahlback and designated as a factor V Leiden mutation.29 It is the consequence of a single point mutation on the factor V gene (adenine for guanine) resulting in factor Va with diminished sensitivity to the natural anticoagulant effect of activated protein C. Approximately 5% of Caucasians in Europe and North America are heterozygous for this genetic defect; lower rates of carrier frequency have been reported among Native American, African, and Asian populations. The heterozygous state carries a 5- to 10-fold increase in lifetime risk for venous thromboembolism, whereas the risk among patients homozygous for this mutation may be increased 80-fold. Factor V Leiden mutation appears to be an important risk factor for venous thromboembolism during pregnancy, in the postpartum period, and during oral contraceptive use.30 Compared with women who do not use oral contraceptives and are not carriers of the factor V mutation, the risk of thrombosis among those with both risk factors is increased approximately 30-fold.31
Another common mutation has been identified in the 3’ untranslated region of the prothrombin gene (substitution of A for G at position 20210) and is present in 2% to 4% of the general population.32 This mutation results in an overproduction of prothrombin, which is otherwise normal. It is associated with a three- to fourfold increased risk of lower extremity venous thrombosis and appears to act in a synergistic manner with other forms of thrombophilia in increasing both the initial and recurrent thrombosis risk.
In clinical practice, factor V Leiden mutation and prothrombin gene mutation are the most common inherited conditions and account for more than half of the cases of inherited thrombophilia-related venous thromboembolism; three other conditions (deficiencies in antithrombin III, protein C, or protein S) account for most of the remainder. Occasionally one may also encounter venous thromboembolism patients who may have other conditions, particularly related to dysfibrinogenemias. It is important to recognize that, when multiple inherited risk factors coexist (such as factor V Leiden and prothrombin gene mutation), the risk of recurrent venous thromboembolism may increase substantially, and lifelong anticoagulation may be necessary in these patients.
PATHOPHYSIOLOGY
Once detached from their point of origin, emboli travel via the systemic venous system, through the right chambers of the heart, and eventually reach the pulmonary arterial system. The physiologic effects and clinical consequences of pulmonary thromboembolism vary widely, ranging from asymptomatic disease to hemodynamic collapse and death. Major factors that determine the outcome include (1) size and location of emboli; (2) coexisting cardiopulmonary diseases; (3) secondary humoral mediator release and vascular hypoxic responses; and (4) the rate of resolution of emboli.
 HEMODYNAMIC CONSEQUENCES
HEMODYNAMIC CONSEQUENCES
Obstruction of the pulmonary vascular bed by embolism acutely increases right ventricular afterload. The normal pulmonary arterial system is a low-pressure system capable of accommodating substantial increases in blood flow with only modest increases in pressure. The thin-walled right ventricle is poorly equipped to generate the pressure necessary to overcome any significant increase in pulmonary vascular resistance. Compensatory mechanisms exist that allow up to 70% obstruction of the pulmonary vascular bed before right ventricular failure develops.33–36
In the absence of pre-existing cardiopulmonary disease, obstruction of less than 20% of the pulmonary vascular bed results in minimal hemodynamic consequences as a result of recruitment and distention of pulmonary vessels. When the degree of pulmonary vascular obstruction exceeds 30% to 40%, modest increases in right ventricular pressure occur, but cardiac output is maintained through an increase in heart rate and myocardial contractility. Compensatory mechanisms begin to fail when the degree of pulmonary artery obstruction exceeds 50% to 60%. Cardiac output begins to fall and right atrial pressure increases dramatically. Mixed venous oxygen saturation falls and a lactic acidosis may develop. With further acute obstruction, the right heart dilates, right ventricular wall tension increases, right ventricular ischemia may develop, the cardiac output falls, and systemic hypotension develops. In patients without prior cardiopulmonary disease, the maximal mean pulmonary artery pressure capable of being generated by the right ventricle appears to be 40 mm Hg (pulmonary artery systolic pressure of approximately 70 mm Hg).34
Other factors may affect the hemodynamic consequences of PE. Patients with pre-existing cardiopulmonary disease often have diminished pulmonary vascular reserve and even a relatively minor embolus may result in significant hemodynamic instability (Fig. 73-3). Alternatively, if the right ventricle has had time (months to years) to hypertrophy in response to a gradual increase in demand (left ventricular disease, idiopathic pulmonary arterial hypertension, chromic thromboembolism, etc.) a significantly higher pulmonary artery pressure may be seen.
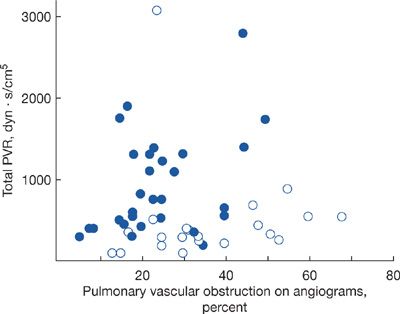
Figure 73-3 Hemodynamic consequences of pulmonary embolism and the underlying state of the pulmonary vasculature. Patients in whom the pulmonary vasculature was previously normal (open circles) develop little increase in pulmonary vascular resistance (PVR) until the clot burden exceeds 50%. In those with antecedent cardiopulmonary disease (solid circles), the PVR increases appreciably with only modest clot burden. (Reproduced with permission from Sharma GV, McIntyre KM, Sharma S, Sasahara AA. Clinical and hemodynamic correlates in pulmonary embolism. Clin Chest Med. 1984;5(3):421–437.)
Several observations suggest that other mechanisms are involved in hemodynamic consequences of acute PE. For example, patients develop only minimal hemodynamic instability during elective lobectomy, pneumonectomy, or even single lung transplantation despite complete and acute interruption of blood supply during cross-clamping. In the experimental setting, cyproheptadine (a nonselective serotonin antagonist) and ketanserin (a selective serotonin antagonist) have been shown to diminish some of the hemodynamic and airway responses that occur after pulmonary embolization. Certain patients develop disproportionately large and fluctuating pulmonary hemodynamic changes in response to relatively small emboli, suggesting that other mechanisms such as reflex vasoconstriction and release of vasoactive compounds may also be involved.37,38
As expected, large or multiple emboli tend to cause more severe symptoms and changes in oxygenation and hemodynamics. Given the large surface area of the peripheral pulmonary vascular bed compared to the central, symptomatic improvement may occur when a large central embolus is fragmented by forces generated by cardiac contractions or even with chest compressions during cardiopulmonary resuscitation. Eventually, the emboli may either resolve by fibrinolysis, or organize and become scar-like tissue that adheres to the vascular endothelium (Fig. 73-4). Recent data suggest that complete resolution is uncommon and that as many as 50% of patients have some residual obstruction 6 months after the embolic event.39,40
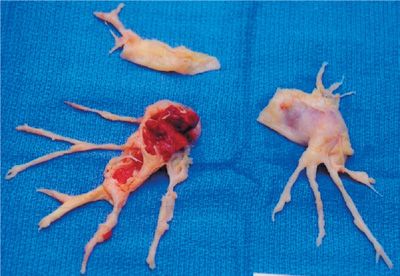
Figure 73-4 Chronic thromboembolic material dissected from pulmonary arteries at pulmonary thromboendarterectomy. Resolution of emboli is occasionally complete but certain patients may be left with significant emboli residua.
 GAS-EXCHANGE ABNORMALITIES
GAS-EXCHANGE ABNORMALITIES
Hypoxemia is the most common immediate physiologic consequence of PE. Obstruction of the pulmonary vasculature prevents systemic venous blood from reaching the pulmonary capillaries of the involved vessels and redirects the blood flow to other parts of the pulmonary vascular bed. This results in an increase in ventilation–perfusion (V/Q) inequality, intrapulmonary shunting, and decreases in the mixed venous O2 level, thereby magnifying the effect of the normal venous admixture.41,42 Further shunting and increase in alveolar dead space can also occur as a result of alveolar hemorrhage or to atelectasis related to loss of surfactant. Despite an increase in alveolar dead space, patients with PE often develop hypocapnia. This is thought to be due to hypoxia-induced intrapulmonary reflex vagal stimulation, with resulting hyperventilation. Finally, embolic events large enough to increase right atrial pressure may result in intracardiac right-to-left shunting through a patent foramen ovale.
One uncommon consequence of PE is pulmonary infarction. Infarction is uncommon because the pulmonary parenchyma has three potential sources of oxygen: the pulmonary arteries, bronchial arteries, and airways. Two of these three sources apparently must be compromised before infarction develops (Fig. 73-5). Therefore, in a patient with no coexisting cardiopulmonary disease, infarction is rare.43 Infarction occurs in approximately 20% of patients with significant cardiac or pulmonary disease that compromise either bronchial arterial flow or airway patency. In patients with left ventricular failure, increased pulmonary venous pressure may decrease bronchial flow and infarction may occur.
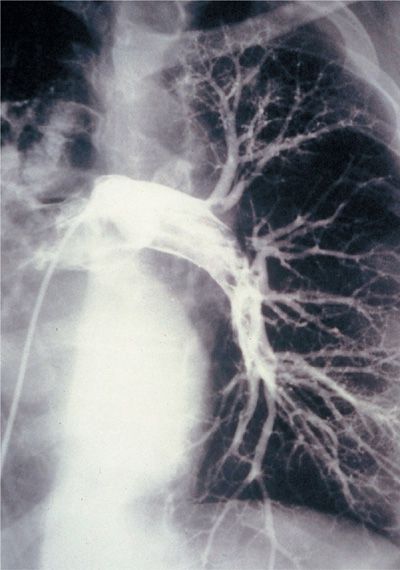
Figure 73-5 Pulmonary angiogram demonstrating thromboembolic obstruction of left pulmonary artery with absent blood flow to lingula and lower lobe. Despite extension obstruction, infarction did not occur as a result of lung’s dual blood supply.
DIAGNOSIS OF PULMONARY EMBOLISM
The diagnostic approach to PE has undergone a fundamental transition over the past decade. V/Q scanning, the mainstay of diagnosis for almost three decades, has been relegated to a secondary role. The prospective investigation of pulmonary embolism diagnosis (PIOPED) trial demonstrated the shortcomings of this technique while providing valuable insight into the diagnostic utility of clinical assessment.44 CT, highly sensitive D-dimer assays, stratification according to clinical assessment, and the application of Bayesian analysis to the diagnostic pathway have become the cornerstones of the current diagnostic approach. What has not changed is the understanding that clinical evidence per se, although capable of raising suspicion of the disease, is incapable of reliably confirming or excluding the diagnosis in the absence of objective testing. Recognition of the clinical signs and symptoms associated with embolism is valuable because clinical findings and clinical suspicion represent an essential first step in the diagnostic pathway.
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
The mainstay for the diagnosis of PE is a high index of suspicion tempered by the reality that most patients with embolism have one or more factors predisposing them to the condition. These predisposing factors need not be major or readily apparent. Advancing age, a period of bed rest, a prolonged air flight, or a minor traumatic injury can result in the development of venous thromboembolism. The absence of a known clinical or thrombophilic predisposition, however, should not dissuade an objective evaluation if the clinical presentation is consistent with embolism.
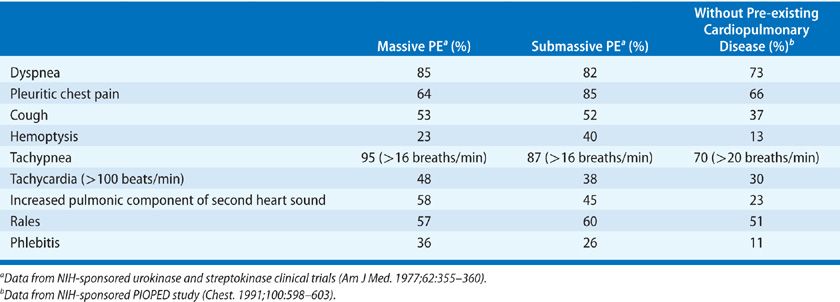
Although a somewhat arbitrary classification (as presenting symptoms and signs of embolism frequently overlap), the presentation of acute PE can be categorized into one of the three clinical syndromes: (1) isolated dyspnea; (2) pleuritic pain or hemoptysis; and (3) circulatory collapse.45,46 Among patients without prior cardiopulmonary disease in the PIOPED study, the syndrome of pleuritic pain or hemoptysis was found to be the most common mode of presentation, occurring in approximately 60% of patients. Isolated dyspnea occurred in approximately 25%, whereas circulatory collapse occurred in 10%.
Two additional modes of presentation are also possible: subclinical clot and chronic, nonresolving or propagating clot. With the increasing use of computed tomographic studies, incidental emboli are occasionally found.47 Typically, these emboli are found in the peripheral segments of the pulmonary arterial vasculature and do not correlate with any clinical symptoms. At this time, the short- and long-term significance of these incidental findings is not clear. In patients who are known to be at high risk of recurrent disease, such as those with inherited thrombophilia and hormonal use or those with poor cardiopulmonary reserve, it is reasonable to consider treatment with anticoagulation or at least the use of more aggressive prophylactic therapies during at-risk situations, such as prolonged hospitalization or air travel.
Complete anatomic resolution of PE appears to be uncommon. When there is sufficient residual pulmonary vascular obstruction, some patients may develop chronic thromboembolic pulmonary hypertension (CTEPH).48 Although exact values for frequencies vary, it is estimated that approximately 0.5% to 1% of patients may develop this condition following an initial, symptomatic episode of PE.49 The diagnosis should be considered even in the absence of a history of acute embolism. Approximately 30% of patients who present with CTEPH do not have a history of precedent acute embolism and are diagnosed during the evaluative process for unexplained dyspnea or pulmonary hypertension.
The most common presenting symptom of acute PE is the sudden onset of dyspnea.45,46 Dyspnea usually occurs over minutes to hours but in approximately 15% occurs over days. Although usually present at rest, dyspnea may only be noted with exertion. It is important to recognize that dyspnea does not occur in approximately 25% of patients ultimately proven to have embolism. Other symptoms include pleuritic chest pain, cough, leg swelling or pain, and hemoptysis. The most common physical finding is unexplained tachypnea (respiratory rate greater than 20/min) present in approximately 70% of patients with embolism. Less frequent physical findings include rales, tachycardia, and an increased pulmonic component of the second heart sound. Fever may develop some hours after the event and often reaches, but rarely exceeds, 38.3°C.50
Obviously, these symptoms and signs are nonspecific (Table 73-2). In the PIOPED study, none of the presenting symptoms or signs with the exception of the presence of rales, a fourth heart sound, and an increased pulmonic component of the second heart sound could differentiate between those with positive and negative angiograms.45
 CLINICAL ASSESSMENT
CLINICAL ASSESSMENT
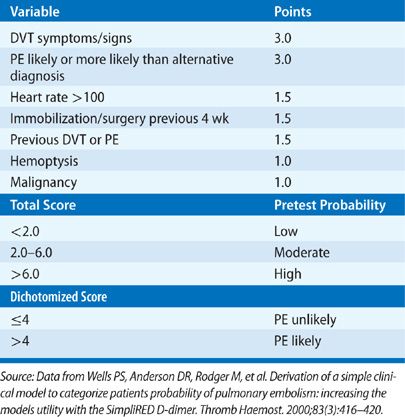
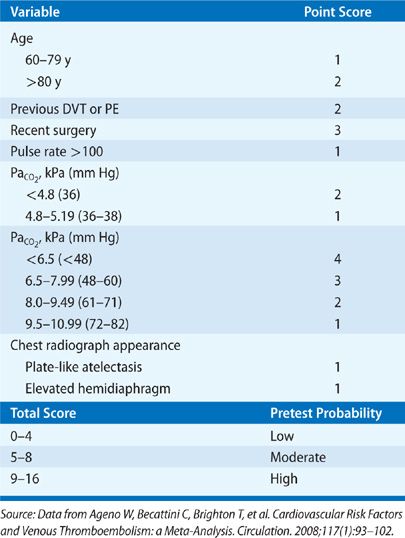
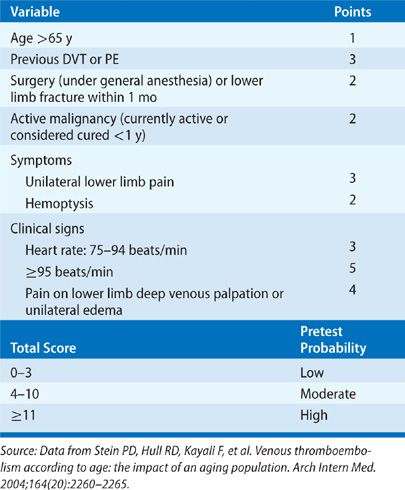
A major advance in the diagnostic approach to PE has been a transition from a purely technique-oriented approach to one that uses Bayesian analysis. In doing so, the pretest probability of the disease, calculated independently of a particular test result using either empiric means or a standardized prediction rule, is calculated. This pretest probability aids in the selection and interpretation of further diagnostic tests to create a posttest probability of the disease. This posttest probability can then be used as a basis for clinical decision making. For PE, a number of such scores have been developed and validated (Tables 73-3–73-5). Wells et al.51 have prospectively tested a rapid seven-item bedside assessment to estimate the clinical pretest probability for PE. An alternative scoring system, the Geneva score, involved seven variables and required gas exchange and radiographic information.52 A revised Geneva score requiring eight clinical variables without gas exchange or radiographic information was validated and published.53 Other clinical decision rules include the PISA rule, the PERC (pulmonary embolism rule-out criteria) rule, and the Charlotte rule.54–56 Although such scoring systems have not proved to be more accurate than clinical assessment, they do provide a method of standardization that compensates for variability in physician experience and judgment.
 LABORATORY FINDINGS
LABORATORY FINDINGS
Routine laboratory testing is not useful in confirming or excluding the diagnosis of PE but may be helpful in suggesting other diagnoses. A modest leukocytosis may accompany embolism but rarely exceeds 20,000/mm3.57
As noted, hypoxemia is common in acute PE although the diagnosis cannot be excluded based upon a normal PO2.58,59 The more massive the obstruction, the more severe the hypoxemia is likely to be. However, many other conditions also cause hypoxemia and, conversely, acute PE does not necessarily cause hypoxemia or even a widening of the (A-a)O2 gradient.58 In the PIOPED trial, no combinations of blood–gas abnormalities were identified that reliably excluded PE. Although most patients with embolism have a low PaO2, low PaCO2, or high P(A-a)O2 gradient, the absence of such abnormal values, alone or in combination, did not exclude PE. Hypercapnia resulting from increased dead space ventilation is rare and appears with PE only in patients with marked antecedent ventilatory limitation or when such limitation has been imposed because the patient is on controlled mechanical ventilation when the embolism occurs.
 ELECTROCARDIOGRAM
ELECTROCARDIOGRAM
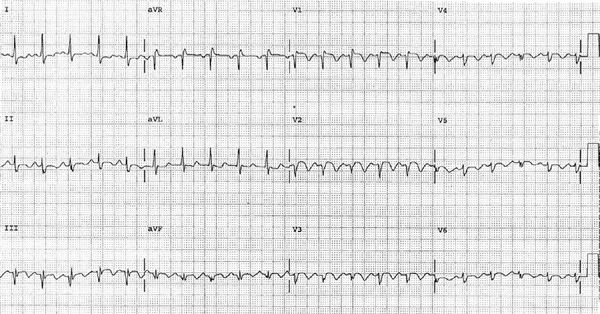
The electrocardiogram is nonspecific in the diagnosis of PE, and its major value may be in identifying other clinical disorders (e.g., acute myocardial infarction and pericarditis) that may be confused with PE. Findings in acute PE are generally nonspecific and include T-wave changes, ST-segment abnormalities, and left- or right-axis deviation (Fig. 73-6).46 Atrial arrhythmias may occur but appear to be more common in patients with underlying cardiopulmonary disease. The S1Q3T3 pattern, commonly considered to be specific for PE, is seen in only a minority of patients. Electrocardiographic findings can offer insight into the extent and hemodynamic consequence of the embolism. The electrocardiogram is rarely normal in the setting of PE associated with right ventricular dysfunction. The presence of an S1Q3T3 pattern, right bundle branch block, or T-wave inversion in leads V1 toV3 in a patient with PE should suggest the presence of right ventricular dysfunction.60,61
Figure 73-6 Electrocardiogram demonstrating findings consistent with embolism including sinus tachycardia, incomplete right bundle branch block, S1Q3T3 pattern, and inverted precordial T waves.
 CHEST RADIOGRAPHY
CHEST RADIOGRAPHY
Most patients with PE have abnormal but nonspecific chest radiographic findings.46,62 Common radiographic findings include atelectasis, pleural effusion, pulmonary infiltrates, and mild elevation of a hemidiaphragm. Classic findings of pulmonary infarction – such as Hampton’s hump or decreased vascularity (Westermark sign) – are suggestive but infrequent. There is some confusion about the diagnostic configuration of infiltrates due to embolism. These infiltrates, although usually abutting a pleural surface, can be of any shape, not necessarily wedge shaped. Although pleural effusions occur in almost half of the patients, the majority of effusions are small and involve only blunting of the costophrenic angle. The main use of the chest radiograph in suspected PE is to exclude alternative diagnostic possibilities such as pneumothorax, which may simulate the disease. A normal chest radiograph in a patient with otherwise unexplained acute tachypnea, dyspnea, or hypoxemia should raise the possibility of PE.
 D-DIMER
D-DIMER
The development of a rapid and accurate blood test capable of diagnosing venous thromboembolism has been the subject of considerable investigative interest. A number of different hemostasiologic markers have been investigated. Of these, D-dimer, alone and in combination with other noninvasive studies has been subjected to the most rigorous clinical evaluation.63,64 D-dimer testing has proven to be highly sensitive but not specific. Increased levels are present in nearly all patients with venous thromboembolism, but also occur in a wide range of other circumstances, including advancing age, pregnancy, trauma, infections, the postoperative period, inflammatory states, and malignancy. Therefore, the role of D-dimer testing is limited to one of venous thromboembolism exclusion. The study is of limited utility in inpatients given the high frequency of positive results in this population.65
Multiple assays for D-dimer have been developed with a range of sensitivities and specificities.66 Highly sensitive assays such as the enzyme-linked immunosorbent assay (ELISA) are capable of excluding venous thromboembolism but are associated with such a high frequency of false-positive results, especially when applied to an inpatient population, as to limit their clinical utility. Less sensitive assays (e.g., latex agglutination, red cell agglutination) lack the ability to exclude venous thromboembolism in isolation but have been used successfully in combination with either a clinical probability estimate or noninvasive diagnostic study. D-dimer testing has been used successfully as part of a number of different diagnostic strategies. Negative results of standardized, highly sensitive assays (ELISA) have proved capable of safely excluding PE in outpatients presenting with a low or intermediate clinical likelihood of the disease. Certain non-ELISA assays are capable of excluding embolism as a stand-alone study in outpatients with a low probability of disease but are more appropriately used in a multibranch diagnostic pathway.
 VENTILATION–PERFUSION SCANNING
VENTILATION–PERFUSION SCANNING
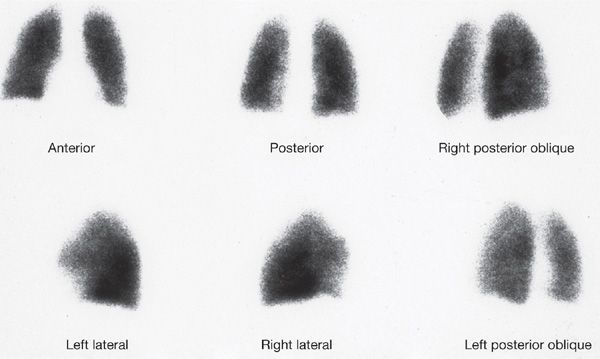
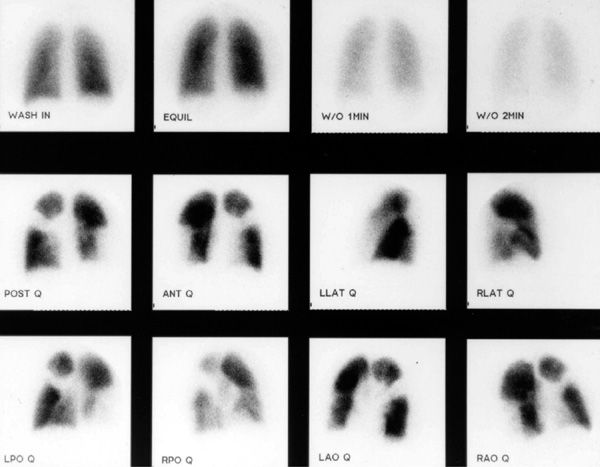
V/Q scanning had been the pivotal diagnostic test for suspected PE for many years but has been replaced by CT imaging. Despite limitations, V/Q lung scanning can provide valuable information if used and interpreted appropriately. The PIOPED trial provided valuable insight into the strengths and limitations of V/Q scanning.44 A negative study is capable of ruling out the diagnosis of PE with the same degree of certainty as a negative pulmonary angiogram and with a higher degree of certainty than can be achieved by a negative CT scan (Fig. 73-7). The positive predictive value of a “high probability” study (one characterized by multiple, segmental-sized, mismatched defects) approximates 88%; when coupled with a high clinical probability of embolism, the positive predictive value increased to 96% (Fig. 73-8). However, only 28% of patients in PIOPED had scans characterized as high probability or normal, the only categories that can be considered definitive. The majority of patients with PE do not have a high probability scan, whereas the majority of those without embolism do not have a normal scan.
Figure 73-7 Normal six-view perfusion scan. Such a scan finding has a negative predictive value equivalent to a negative pulmonary angiogram and higher than that of a negative computed tomographic study.
Figure 73-8 “High probability” ventilation/perfusion scan demonstrating normal ventilation and multiple mismatched segmental and larger defects.
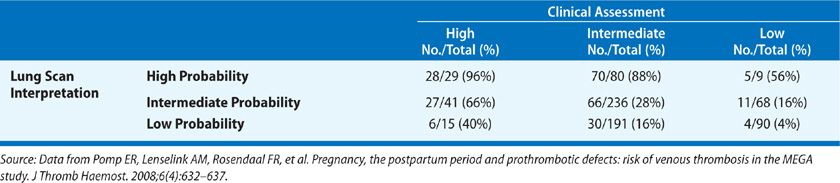
The PIOPED study also undertook to correlate the clinical impression of the likelihood of PE with the interpretation of the lung scan (Table 73-6). When interpretation of the lung scan and clinical assessment were concordant (both high and low probability), diagnostic accuracy was greater than that of the lung scan alone. In contrast, when interpretation of the lung scan and clinical assessment were discordant, the predictive value of the lung scan was decreased. In as many as two-thirds of patients suspected of PE, the combination of the lung scan and clinical assessment were either discordant or indeterminate and failed to diagnose or exclude PE.
TABLE 73-6 Prevalence of Pulmonary Embolism in PIOPED: Value of Correlating Lung Scan Interpretation with Clinical Assessment
There are certain situations in which V/Q scanning may be preferred over CT-pulmonary angiography (CT-PA). IV contrast is not required for V/Q scanning making it a more desirable option in patients with renal dysfunction or a severe iodinated contrast allergy. In addition, with a portable gamma scintillation camera the perfusion portion of the study can be performed at the bedside, which may be a major advantage in a critically ill patient for whom transportation to the CT scanner may be deemed too high risk. The role of V/Q scanning versus CT-PA in pregnancy remains unsettled but V/Q scanning appears to offer similar diagnostic performance in this setting with significantly lower levels of maternal radiation.67
 ECHOCARDIOGRAM
ECHOCARDIOGRAM
The overall sensitivity of transthoracic echocardiography in PE approximates 50%.68 Under appropriate clinical circumstances, the detection of unexplained right ventricular volume or pressure overload should suggest the possibility of PE and lead to confirmatory testing. Transthoracic echocardiography has emerged as a potentially important tool for risk assessment and treatment guidance in patients with acute PE. The presence of right ventricular dysfunction on a baseline echocardiogram in normotensive patients appears to represent an independent predictor of an adverse outcome or early death.69,70 Properly performed transesophageal echocardiography has demonstrated sensitivity and specificity exceeding 90% in the detection of proximal emboli involving the pulmonary trunk and the right and left main pulmonary arteries.71 Echocardiography also may prove valuable in the evaluation of competing diagnostic possibilities such as right ventricular infarction, endocarditis, pericardial tamponade, and aortic dissection in patients with unexplained shock and evidence of elevated central venous pressure.
 LOWER EXTREMITY EVALUATION
LOWER EXTREMITY EVALUATION
Duplex ultrasonography, which refers to the combination of Doppler venous flow detection and real-time B-mode imaging, has assumed a central role in the noninvasive diagnosis of symptomatic lower extremity DVT.72 A number of criteria are used to diagnose DVT, the most reliable of which is noncompressibility of a venous segment. Secondary, less reliable criteria include the presence of echogenic material within the venous lumen, loss of phasicity with respiration, attenuated increase in venous diameter in response to Valsalva, and lack of augmentation of flow in response to calf compression. The absence of an echogenic luminal mass cannot be considered useful in excluding the diagnosis of venous thrombosis because acute thrombus may not demonstrate echogenicity. Multiple studies over the past decade have demonstrated sensitivities and specificities exceeding 95% in symptomatic patients with proximal DVT.
While duplex ultrasonography is highly sensitive and specific in the diagnosis of DVT in symptomatic patients, its accuracy in the diagnosis of asymptomatic DVT is less clear. A meta-analysis of studies comparing ultrasound to contrast venography in asymptomatic patients found that ultrasound was accurate for the detection of asymptomatic proximal DVT, but the data was limited almost entirely limited to postoperative orthopedics patients.73 Approximately 30% to 40% of patients with PE will also have signs and/or symptoms of DVT and 60% to 80% will have evidence of proximal DVT when subject to duplex ultrasonography. However, given that the overwhelming majority of patients with suspected PE will not prove to have that diagnosis, duplex ultrasonography as the initial diagnostic study should be reserved for those patients with clinical evidence of DVT and for special populations in which avoidance of radiation or contrast material is preferred.74–77
The role of CT venography as a stand-alone test for DVT is limited. CT venography appears to be comparable to ultrasonography with respect to sensitivity and specificity, but it requires contrast injection with its associated risks and radiation exposure. A potential advantage of CT venography is the ability to visualize the pelvic veins and vena cava. The concept of combined CT-PA and venography is attractive. Such an approach would provide visualization of the embolus and its source in a single study as well as potentially increase diagnostic yield in comparison with the use of CT-PA alone.78 However, the absolute increase in diagnostic yield appears to be modest and comes at the cost of increased expense, substantial pelvic radiation exposure, and the risk of hemorrhagic complications from providing anticoagulation to patients with false-positive studies.
 MAGNETIC RESONANCE IMAGING
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) techniques for detecting DVT and PE have been investigated. The PIOPED III trial was the largest, multicenter study designed to assess magnetic resonance angiography (MRA) for the diagnosis of PE.79 Three hundred and seventy-one patients were enrolled in whom PE was confirmed or excluded by CT-PA or V/Q. Approximately 25% of studies were technically inadequate and could not be interpreted. However, this seemed to vary by site (11%–52%) suggesting that some sites may have had more experience and were able to limit their number of inadequate scans. The sensitivity and specificity of MRA among patients with technically adequate scans was 78% and 99%, respectively. These figures fell considerably when the technically inadequate scans were included in the calculations. Sensitivity for PE involving the main or lobar pulmonary arteries was only 79%.
There are patients in whom CT-PA may not be possible due to IV contrast allergy or in whom avoidance of radiation is preferred (e.g., pregnancy). In these patients it seems that MRA may be a reasonable alternative. The risk of gadolinium-related nephrogenic systemic fibrosis has dampened the initial enthusiasm for MRI scanning in patients with renal insufficiency.80
 COMPUTED TOMOGRAPHY
COMPUTED TOMOGRAPHY
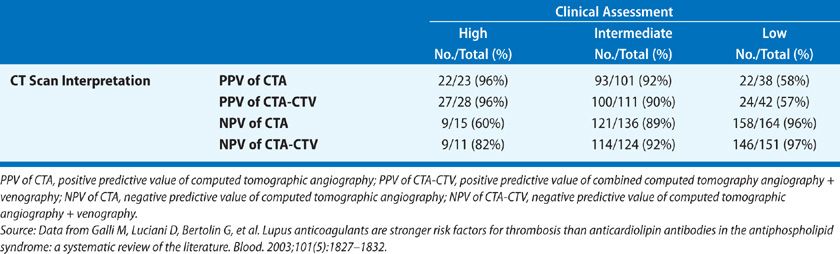
CT-PA has become the first-line imaging test for PE (Fig. 73-9). CT technology has evolved from single detector scanners to 64-multidetector-row CT (MDCT) scanners. Using the latest generation scanners, visualization of the entire chest with submillimeter resolution extending to the sixth generation arteries can now be performed within a single breath hold. The PIOPED II trial, which used predominantly 4-MDCT technology and a composite reference standard, demonstrated sensitivity for the diagnosis of PE of 83%, specificity of 96%, positive predictive value of 86%, and negative predictive value of 97% (Table 73-7).46 The predictive value of CT-PA varied substantially when clinical assessment was taken into account, with the major variance occurring when there was discordance between the clinical assessment and CT-PA finding. Both the positive predictive value in patients with a low clinical probability and the negative predictive value in those with a high clinical probability were in the range of 60%. At the present time, CT can be considered confirmatory in excluding embolism in patients with a low or intermediate likelihood of disease and confirming embolism in patients with intermediate or high probability of disease. When discordance exists between the clinical assessment and CT findings, additional studies should be considered.
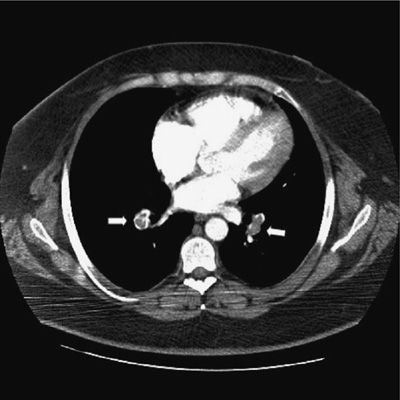
Figure 73-9 Computed tomographic angiogram demonstrating nearly occlusive thrombus in both lower lobe pulmonary arteries (arrows).
TABLE 73-7 Prevalence of Pulmonary Embolism in PIOPED II: Value of Correlating CT Interpretation with Clinical Assessment
Concerns have been raised that the widespread utilization of CT-PA is resulting in the overdiagnosis of PE, defined as diagnosing clinically insignificant disease. The highest level of evidence thus far is a randomized controlled trial by Anderson et al.81 of pulmonary CT-PA versus V/Q scan for suspected PE that showed comparable mortality and false-negative rates for the two imaging modalities with a 51% higher rate of PE diagnosis for CT. It has been suggested, based on the known natural history of subsegmental embolism, that patients with isolated subsegmental PE do not necessarily need to be treated with anticoagulants provided they meet the following criteria: (1) good cardiopulmonary reserve; (2) no evidence of DVT with serial lower extremity studies; (3) a transient (reversible) major risk factor for PE that is no longer present; (4) no history of central venous catheterization and no history of atrial fibrillation; and (5) a compliant and trustworthy patient who would return for serial noninvasive lower extremity studies.82 Concerns have also been raised that CT-PA, as a result of its easy availability, is being overutilized in outpatients without adequate patient screening through validated methods such as the Wells criteria/D-dimer model or the PERC.55,83
 CONVENTIONAL PULMONARY ANGIOGRAPHY
CONVENTIONAL PULMONARY ANGIOGRAPHY
Prior to widespread acceptance of CT-PA, pulmonary angiography was considered the accepted “gold standard” for PE diagnosis although it had a number of limitations (Fig. 73-10). It requires expertise in study performance and interpretation. It is invasive and has associated risks, although published studies suggest that the use of modern techniques and contrast materials has reduced the risk well below its lingering perception. Pulmonary angiography can be performed quite safely if certain safeguards are observed and experienced personnel are involved. In the 1111 angiograms performed in the original PIOPED trial, pulmonary angiography was nondiagnostic in 3% of patients and associated with a major complication rate of 1% and death rate of 0.5%.84 Major complications occurred more frequently among patients sent for angiography from the medical intensive care unit than in patients from elsewhere (4% vs. 1%). The procedure has other limitations. One is accessibility and the need for transportation. The other limitation is interpretation. Interpretation of pulmonary angiograms is heavily influenced by three factors: the location of the thromboembolic obstruction, the quality of the images, and the experience of the interpreters. Only two angiographic findings are diagnostic of acute embolism: a filling defect and abrupt cutoff of a vessel. Technical adequacy of the angiogram is critical to accurate identification of both. Flow artifacts can falsely suggest a filling defect. It is essential that good vessel opacification be obtained and that the filling defects be identified as real on a sequence of films.
Figure 73-10 Conventional contrast pulmonary angiogram demonstrating extensive embolus within the left main pulmonary artery and extending into lobar branches.
Angiography is reserved for the small subset of patients in whom the diagnosis of PE cannot be established or excluded by less invasive means and for the evaluation of suspected chronic thromboembolic disease.
DIAGNOSTIC APPROACH
The diagnostic approach to PE should be targeted toward the patient population being studied (Figs. 73-11 and 73-12). For outpatients, the use of a clinical prediction rule coupled with D-dimer testing or the use of the PERC criteria can substantially reduce the number of imaging studies performed. In the outpatient setting, D-dimer results would not alter the need for an objective imaging study in patients with a high probability of PE. In patients with a low or intermediate clinical likelihood of PE, a negative D-dimer study is sufficient to exclude the diagnosis, assuming a highly sensitive assay is used. An imaging study should be performed in all patients with a high probability of disease as well as those with a low or intermediate probability whose D-dimer tests are positive.
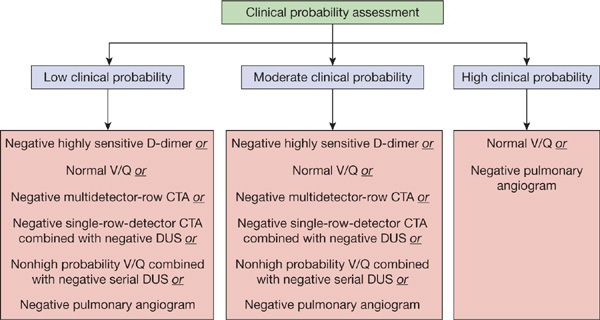
Figure 73-11 Current diagnostic strategies capable of excluding diagnosis of pulmonary embolism.
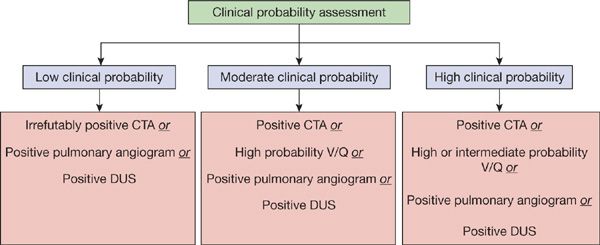
Figure 73-12 Current diagnostic strategies capable of confirming diagnosis of pulmonary embolism.
Patients with signs or symptoms of lower extremity DVT should initially undergo lower extremity duplex ultrasonography. A positive lower extremity study, although not proving PE has occurred, has the same therapeutic implications. Although such an approach will yield a confirmatory study in only about 20% of patients suspected of having PE, it avoids the cost and radiation exposure associated with CT-PA. A negative study is insufficient to exclude the possibility of PE.77
In patients without lower extremity symptoms or signs who have a high or intermediate clinical probability of PE, a positive CT-PA confirms the diagnosis. In patients with a low or intermediate clinical probability, a negative CT-PA excludes the diagnosis. The only patients who may require additional testing (duplex ultrasonography and/or conventional angiography) are those in whom the clinical assessment and CT findings are discordant (low clinical probability and positive CT scan or high clinical probability and negative CT scan) unless the CT scan is of adequate quality and demonstrates evidence of PE in the main or lobar arteries in a patient with a low clinical probability assessment.
An approach utilizing V/Q scanning as the primary imaging tool can be used in settings such as pregnancy, contrast allergy, or renal insufficiency. This approach should be reserved for those with normal chest radiographs and no history of chronic pulmonary disease. Lower extremity evaluation should be considered before chest imaging given the likelihood that the V/Q scan will not be diagnostic. A negative V/Q scan is capable of excluding the diagnosis regardless of the clinical assessment as does a low probability scan in conjunction with a low clinical suspicion. A high probability scan is capable of confirming the diagnosis in patients with a high clinical suspicion. All other circumstances (high clinical probability with low or intermediate V/Q result, intermediate clinical probability regardless of V/Q result, and low clinical probability with a high or intermediate V/Q scan result) require additional testing.44
Whatever approach is undertaken, the treating physician should be aware of the type of D-dimer assay, discriminant value of that particular assay, and generation of CT scanner used. “Negative” findings on a low-sensitivity D-dimer assay or a single-row CT scanner have very different implications than similar findings using a highly sensitive D-dimer assay or 64-MDCT scanner.
The role of D-dimer testing and clinical assessment in hospitalized patients is limited. A substantial proportion of hospitalized patients have a positive highly sensitive D-dimer result related to an active inflammatory process, malignancy, recent surgery, liver disease, and even advancing age.85 In a recent study that evaluated the use of the Wells prediction rule in conjunction with a latex agglutination or rapid ELISA D-dimer assay in hospitalized patients, only 10% of over 600 patients tested fell into the low probability category.85 Furthermore, comorbid conditions affect the clinical likelihood assessment. Therefore, a far higher proportion of hospitalized patients require an imaging study to confirm or exclude the diagnosis of venous thromboembolism. In patients with limited cardiopulmonary reserve, high clinical probability assessment, and negative CT scan, pulmonary angiography should be considered given the potentially fatal consequences of a recurrent embolic event. In patients with adequate cardiopulmonary reserve under the same circumstances, a strategy incorporating sequential lower extremity evaluation can be undertaken.
TREATMENT
Management of acute PE consists of a systematic approach that involves early intervention, patient risk stratification, selection of therapy, and determination of treatment duration. The goals of therapy in PE are severalfold—to assure adequate oxygenation, provide hemodynamic support, and prevent thrombus propagation and embolic recurrence.
When a diagnosis of venous thromboembolism is suspected, empiric treatment should be considered until the diagnosis is either objectively excluded or confirmed. Given the ready availability of rapid D-dimer assays and CT, diagnostic confirmation or exclusion should require a relatively short period of time. Early empiric treatment should be initiated if diagnostic tests are not readily available. An exception can be made in those patients with a low clinical likelihood of disease, adequate cardiopulmonary reserve, and a high risk of bleeding complications.
The availability of low–molecular-weight heparin (LMWH) potentially allows carefully selected patients to be managed in the outpatient setting as demonstrated in the recently published study.86 Although there are good data to support treating uncomplicated cases of DVT entirely in the outpatient setting, most physicians still advocate a short period of hospitalization in patients with newly diagnosed acute PE. Hospitalization should be mandatory for older patients who may have less cardiopulmonary reserve, or significant coexisting illnesses, or those who may not be able to follow instructions or have adequate follow-up. Other indications for hospitalization include hypoxemia, hypotension, hemodynamic instability, or sufficient renal disease to contraindicate the use of LMWH or a factor Xa inhibitor.
 HEPARIN
HEPARIN
Anticoagulation with heparin remains the standard initial therapy. The major anticoagulant effect of heparin is to reduce thrombus propagation and prevent embolic recurrence. Choices include either intravenous unfractionated heparin (UFH) or subcutaneous LMWH preparations. Given that failure to achieve rapid therapeutic levels of anticoagulation appears to be associated with an increased recurrence rate, it is reasonable to attempt to ensure adequate anticoagulation as soon as possible.87
Physician practices in the administration of intravenous unfractionated heparin have often resulted in substantial delays before adequate prolongation of the activated partial thromboplastin time (aPTT) is achieved. To overcome these problems, standardized protocols for unfractionated heparin administration and monitoring have been recommended. One commonly employed dosing regimen using an initial intravenous bolus of 80 units of heparin per kilogram followed by a continuous infusion initiated at 18 U/kg/h has been demonstrated to reach therapeutic thresholds more quickly than regimens using fixed dosing.88 The heparin drip is adjusted based on monitoring of the aPTT, drawn 6 hours after the initial bolus dose, then 6 hours after each dose adjustment, with a target aPTT ratio of 1.5 to 2.5. Because attempts to straddle the lower therapeutic range may result in periods of inadequate anticoagulation, it is prudent to maintain the ratio in the upper range of the recommended target.
More recently, an approach using a fixed dose of subcutaneous unfractionated heparin without aPTT monitoring, administered as an initial dose of 333 U/kg followed by a dose of 250 U/kg every 12 hours, has been demonstrated to be as safe and effective as LMWH in patients presenting with DVT and PE.89
With the exception of special circumstances, LMWH preparations have displaced unfractionated heparin as the anticoagulant of choice in uncomplicated venous thromboembolism including PE.90 Situations in which the use of UFH is appropriate include renal insufficiency, extremes of body weight, and circumstances in which a rapid adjustment or reversal of anticoagulation is needed, such as women in the late stage of pregnancy who may need Cesarean sections, patients with recent surgery or recent history of bleeding, and hemodynamically unstable patients with venous thromboembolism who may need surgical procedures such as emergency embolectomy.
Available evidence suggests that LMWH is at least as effective as unfractionated heparin in treating acute PE.91 Advantages of LMWH compared with UFH include (1) longer half-life and ease of use; (2) ability to consistently achieve early therapeutic anticoagulation; (3) no need to monitor anticoagulant effects; and (4) reduced incidence of major bleeding complications. There are few data comparing different LMWH preparations. Even though there are differences in their Food and Drug Administration (FDA)-approved indications in the United States, it is not clear if their actions differ significantly.
In general, therapeutic monitoring is not needed with LMWH, but there are situations where the therapeutic effects may be less predictable and monitoring with anti-Xa levels is indicated. Typical examples include (1) patients with antiphospholipid antibodies or other circulating anticoagulants who have elevated baseline aPTT; (2) extremes of body weight (less than 40 kg and greater than 150 kg); (3) significant renal disease (creatinine clearance less than 30 mL/min); (4) pregnancy; and (5) unexplained bleeding or recurrent thrombosis during therapy. A therapeutic target range for peak anti-Xa levels ranges from 0.6 to 1.0 IU/mL, 4 hours after administration. The target range for peak anti-Xa levels with once daily enoxaparin is likely to be greater than1.0 IU/mL whereas it is greater than 0.85 IU/mL with tinzaparin and 1.3 IU/mL and 1.05 IU/mL with nadroparin and dalteparin, respectively.92
 FACTOR XA INHIBITORS
FACTOR XA INHIBITORS
Fondaparinux, a synthetic pentasaccharide, represented the first in a new class of antithrombotic agents. Unlike unfractionated heparin and LMWH, the antithrombotic properties of fondaparinux are selective for factor Xa. By binding rapidly and strongly to antithrombin, fondaparinux catalyzes specifically the inhibition of factor Xa, which results in inhibition of thrombin generation. It does not bind to other plasma components or platelets, has a half-life of approximately 17 hours, and is excreted almost completely by the kidneys. Fondaparinux was shown to be as effective and safe as intravenous unfractionated heparin in a large, open-label study.93 It has been approved for prophylaxis in patients undergoing hip, knee, and abdominal surgery as well as for treatment of DVT and PE in conjunction with warfarin.
Rivaroxaban represents the first in a new generation of oral factor Xa inhibitors. In a large, open-label randomized study, rivaroxaban proved noninferior to conventional therapy (LMWH followed by a vitamin K antagonist) in patients with symptomatic DVT.94 Patients with acute PE were excluded from the acute phase of the study. Although not specifically designed to determine efficacy in acute PE, the incidence of subsequent PE was equivalent between the two study groups. In a subsequent study involving 4832 patients, a fixed-dose regimen of rivaroxaban alone was noninferior to standard therapy for the initial and long-term treatment of PE with or without DVT.95 Ongoing trials are underway evaluating the efficacy and safety of other oral factor Xa inhibitors, including apixaban and edoxaban in venous thromboembolism.96
Intravenously administered direct thrombin inhibitors (lepirudin, argatroban) represent another class of anticoagulant agents that have been approved for the management of patients with venous thromboembolism in the setting of heparin-induced thrombocytopenia (HIT).97 Their mechanism of action differs from that of heparin and the synthetic pentasaccharides in that they directly inhibit the active site of thrombin and do not require interaction with antithrombin to produce an anticoagulant effect. Argatroban is a synthetic agent derived from arginine. It has a half-life of approximately 45 minutes and is cleared by the liver. Lepirudin is a recombinant polypeptide similar to hirudin. It has a half-life of 40 to 60 minutes and is cleared by the kidneys. Both agents are administered by continuous intravenous infusion and dose adjustments made with monitoring of the aPTT. Both agents affect the international normalized ratio (INR), thereby complicating the transition to oral warfarin therapy. Although demonstrating promise, data supporting the use of fondaparinux in the management of HIT is far less robust than that of the intravenous direct thrombin inhibitors.98 Based on its size, the drug is less immunogenic than unfractionated heparin or LMWH. Fondaparinux has not been approved by the FDA for this indication.
 THROMBOLYTIC THERAPY
THROMBOLYTIC THERAPY
Unlike anticoagulants, thrombolytic drugs cause direct lysis of thrombi by increasing plasmin production through plasminogen activation. The potential benefits, however, are often offset by the relatively high incidence of hemorrhagic complications.
Multiple thrombolytic agents are available. Those most studied for the management of acute PE include streptokinase, alteplase (rt-PA), and urokinase, all of which are FDA approved for use in the United States. The PEITHO (pulmonary embolism thrombolysis) trial, a large multicenter study designed to enroll 1000 patients, is currently underway and will evaluate the efficacy and safety of tenecteplase in normotensive patients with evidence of right ventricular (RV) dysfunction.99
The exact role of thrombolytic agents in acute PE remains controversial. While thrombolytic therapy does appear to accelerate the rate of thrombolysis, there is no convincing evidence to suggest that it decreases mortality, increases the ultimate extent of embolic resolution when measured at 7 days, reduces thromboembolic recurrence rates, improves symptomatic outcome, or decreases the incidence of thromboembolic pulmonary hypertension.100 The one issue about which there can be little controversy is that the use of thrombolytic agents is associated with a substantially increased risk of bleeding, including intracranial hemorrhage. Intracranial hemorrhage has occurred in 0.5% to 3.0% of patients treated with thrombolytic agents in trials evaluating the use of these agents in both PE and myocardial infarction.101
Based on these data, and assuming there is no contraindication to its use, the use of thrombolytic therapy in PE appears to be appropriate when an accelerated rate of thrombolysis may be considered lifesaving. Specifically, this applies to patients with PE who present with hemodynamic compromise (in whom the mortality rate approaches 30%), patients who develop hemodynamic compromise during conventional therapy with heparin, and patients with PE associated with intracavitary right heart thrombi.36
The role of thrombolytic therapy in patients with anatomically massive embolism or echocardiographic evidence of right ventricular dysfunction in the absence of systemic hypotension is less well defined. Risk stratification strategies using echocardiography, troponin levels, or brain natriuretic peptide (BNP) levels are currently under investigation and may help resolve this area of controversy.102 At the present time, the finding of right ventricular dysfunction on echocardiography in the absence of hemodynamic instability would not appear to serve as a justification for the routine use of thrombolytic therapy. Approximately 40% to 50% of patients with symptomatic PE have echocardiographic evidence of right ventricular dysfunction.103 Clinical scoring systems have also been constructed capable of estimating 30-day mortality rates in patients with acute PE (Table 73-8).104,105 However, these scoring systems appear best suited in identifying patients with a low mortality risk who may be suitable for home management rather than those with a sufficiently high mortality risk to justify the use of thrombolytic therapy.