Pulmonary Thromboembolectomy and Thromboendarterectomy
Stuart W. Jamieson
Introduction
Pulmonary embolectomy is an uncommonly applied operation for life-threatening hemodynamic instability from occlusive acute pulmonary emboli. The application of this procedure has become less frequent since the recognition of the fact that generally, after acute massive pulmonary embolism, patients who survive transfer to hospital and the operating room will likely survive without operative intervention anyway. In addition, currently, many patients will undergo fibrinolytic therapy, or percutaneous thrombectomy or lysis in the cardiac catheterization laboratory. Nonetheless, the operative technique is described here for those rare cases that require operative treatment because of particular urgency or unavailability of alternative therapies.
Pulmonary thromboendarterectomy (PTE), on the other hand, is becoming a more commonly applied procedure with the increased recognition that pulmonary hypertension and right ventricular failure due to thromboembolic disease is more prevalent than is generally appreciated. Medical management is limited to the treatment of right heart failure, and is palliative only. Surgical treatment is curative and, with current techniques, has a low mortality and morbidity.
Acute Pulmonary Embolectomy
The mortality rate of massive acute pulmonary embolism is approximately 10%. Most (75% to 90%) patients who die of pulmonary emboli do so within the first few hours of the primary event. In those with sufficient cardiopulmonary reserve and right ventricular strength to survive the initial few hours, autolysis of emboli will occur over the
next few days, with complete resolution occurring within 3 to 4 weeks. Since in the majority of patients the pulmonary emboli continue to resolve, surgical embolectomy is not necessary for survival except in a minority of patients.
next few days, with complete resolution occurring within 3 to 4 weeks. Since in the majority of patients the pulmonary emboli continue to resolve, surgical embolectomy is not necessary for survival except in a minority of patients.
Advances in fibrinolytic therapy and procedures in the cardiac catheterization laboratory, which include fragmentation or suction of the thrombus, have added to the therapeutic armamentarium for the treatment of acute pulmonary embolism and lessened the application of surgical embolectomy.
The operation is indicated in those patients demonstrating severe hemodynamic instability after massive acute embolism in a setting where intervention in the catheterization laboratory with lysis or suction of the emboli, usually in combination with intravenous lytic therapy, is not available or practical. The operation is contraindicated when the patient is hemodynamically stable.
The first priority after sudden collapse of any patient is to establish adequate ventilation and circulation. This may require intubation and mechanical ventilation. In patients after acute major pulmonary embolism with hypoxia and mild hypotension (systolic arterial pressure maintained above 90 mm Hg), without cardiac arrest or sustained low cardiac output and cardiogenic shock, the aim should be to definitively establish the diagnosis and to attempt pharmacologic therapy and possibly remove the embolic material by catheter suction or fragmentation.
Inotropes and pressors are used to help stabilize the patient’s hemodynamics. If the patient’s circulation can be stabilized, intravenous heparin is started with the goal of preventing the propagation and formation of new thromboemboli, though this will not dissolve the existing clot. In most instances the patient’s own fibrinolytic system will lyse fresh thrombi over a period of weeks.
Mechanical removal of pulmonary thrombi may be achieved by a catheter device inserted under local anesthesia into the femoral or jugular veins. Fragmentation, suction, or snaring of thrombus have been used. Successful extraction of clot with a meaningful reduction in pulmonary arterial pressure can be achieved in a majority of patients. In the absence of contraindications, fibrinolytic therapy is then added (streptokinase, urokinase, or recombinant tissue plasminogen activator [rt-PA]) to increase the rate of lysis of fresh thrombi, though bleeding complications with these agents occurs in approximately 20% of patients.
If hemodynamic stability cannot be achieved, then urgent operation is indicated. Of those who do not survive a massive acute pulmonary embolus, approximately 10% will die within the first hour, 50% within 2 hours, and 85% within 6 hours. The availability of the necessary equipment, personnel, and experience will determine the therapeutic options.
Since a clinical diagnosis of pulmonary embolus is often wrong, transesophageal echocardiography and color Doppler mapping should be used to confirm the diagnosis even if the patient has been urgently transferred to the operating room. Transesophageal echocardiography will show increased right ventricular volume, poor right ventricular contractility, and tricuspid regurgitation. Clot may be seen trapped within the right atrium or ventricle, and sometimes visualized in the main pulmonary artery.
The patient should be prepared and draped ready for immediate incision prior to anesthetic induction. Careful anesthetic induction should be administered to avoid further cardiovascular collapse after vasodilatation.
A midline sternotomy incision is used and cardiopulmonary bypass is initiated as soon as possible. The heart is arrested with cold cardioplegic solution. The
main pulmonary artery is then opened 1 cm distal to the pulmonary valve, and the incision extended into the proximal left pulmonary artery. Stay sutures are placed (Fig. 41.1).
main pulmonary artery is then opened 1 cm distal to the pulmonary valve, and the incision extended into the proximal left pulmonary artery. Stay sutures are placed (Fig. 41.1).
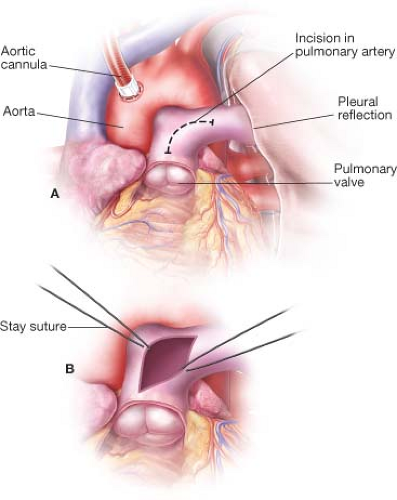 Figure 41.1 The incision in the main pulmonary artery begins 1 cm. distal to the pulmonary valve and extends into the left pulmonary artery. |
Forceps and suction are then used to remove as much thrombus as possible from both main pulmonary arteries. The pleural spaces are entered, and each lung is gently manually compressed to dislodge smaller clots into the larger vessels. If a sterile pediatric bronchoscope is available, this is helpful in locating and removing thrombi in the tertiary and quaternary pulmonary vessels. The pulmonary arteriotomy is closed with a double layer of running polypropylene, and the patient weaned from bypass in standard fashion.
Consideration should be given to the placement of an inferior vena cava filter before closing the chest. This can be done through the right atrium with radiographic guidance. The Greenfield filter is the most commonly used and is associated with a lifetime recurrent embolism rate of 5% and a 97% patency rate. Anticoagulation for 6 months is recommended after any pulmonary embolic event, provided there are no contraindications to its use.
Mortality rates for emergency pulmonary thromboembolectomy vary widely and are between 40% and 90%. The outcome depends largely upon the preoperative condition and circulatory status of the patient. If cardiac arrest has occurred, mortality and complications are obviously higher than in a patient in which cardiopulmonary bypass can be established with relative hemodynamic stability. Recurrent embolism is uncommon, and approximately 80% of survivors maintain normal pulmonary arterial pressures and exercise tolerance. In these patients postoperative angiograms are normal, or show less than 10% obstructed vessels.
Chronic Pulmonary Endarterectomy
In a significant minority (approximately 5%) of patients who survive acute pulmonary embolic episodes, the clot does not resolve completely. Only partial lysis occurs, with the result that thrombus is eventually layered into the pulmonary artery wall and becomes fibrotic. In addition there may be residual webs or bands across the lumen of the smaller vessels. The progressive occlusion of the pulmonary vasculature results in pulmonary hypertension. It should be noted that in many (up to 50%) subjects with chronic pulmonary hypertension from thromboembolic disease no history of pulmonary emboli can be obtained.
The reasons for the failure of emboli to dissolve are incompletely understood, and may be a combination of factors. It may be related to repetitive thrombi, or embolization of already partially fibrotic material. The volume of acute embolic material may simply overwhelm the lytic mechanisms, or a total occlusion of a major arterial branch may prevent lytic material from reaching, and thus dissolving, the embolus completely. The lytic mechanisms themselves may be abnormal, and some patients may actually have a propensity for thrombus, or a hypercoagulable state.
Chronic indwelling lines, pacemaker leads, and central catheters are sometimes associated with pulmonary emboli. Patients with ventriculoatrial shunts also sometimes present with pulmonary hypertension, and in these patients it seems that an additional factor is involved, perhaps direct chemical irritation of the pulmonary vascular bed.
Whatever the predisposing factors to residual thrombus within the vessels, the final genesis of the resultant pulmonary vascular hypertension may be complex. The increased pressure and flow as a result of redirected pulmonary blood flow in the previously normal pulmonary vascular bed can create a vasculopathy in the small precapillary blood vessels, similar to that seen in Eisenmenger syndrome. These changes are not operable or reversible. With the current success and attendant low mortality with the pulmonary endarterectomy operation we thus advise earlier operation, before these changes can occur.
The prognosis for all patients with pulmonary hypertension is poor. For patients with chronic pulmonary hypertension due to thromboembolic disease, survival is proportional to the degree of hypertension. If the mean pulmonary pressure at presentation is over 50 mm Hg, the 2-year survival rate is 20% and the 5-year survival 10%. Surgical therapy offers a vastly improved prognosis.
Pulmonary embolization uncommonly results in tissue necrosis because of the bronchial circulation. Surgical endarterectomy, thus, will allow distal pulmonary tissue to be used once more in gas exchange.
There have been three major indications for considering pulmonary endarterectomy: Hemodynamic, respiratory, and prophylactic. The hemodynamic goal is to prevent or ameliorate right ventricular compromise due to pulmonary hypertension. The respiratory objective is to improve respiratory function by removing a large ventilated, but unperfused, physiologic dead space. The prophylactic goal is to prevent progressive right ventricular dysfunction, or retrograde extension of the obstruction, which might result in further cardiorespiratory deterioration or death. Our subsequent experience has added another prophylactic goal; the prevention of secondary arteriopathic changes in the remaining open vessels, as discussed above.
Symptoms and signs: Patients may be asymptomatic until symptoms of dyspnea, exercise intolerance, or right heart failure develop. Because of the large area of the pulmonary vascular bed, generally more than 60% of the vasculature must be occluded before pulmonary hypertension occurs at rest. With exercise and the attendant increase in cardiac output, however, even with lesser degrees of occlusion, the pulmonary artery pressures will increase and the increase in pulmonary blood flow may be associated with a widening of the alveolar–arterial oxygen tension gradient, with subsequent hypoxemia.
The principal symptom in patients is therefore progressive exercise intolerance, typically portrayed as exertional breathlessness. It is not until the relatively late stages of the disease when the signs of right heart failure become obvious that the diagnosis can easily be made. In the late stages the patient will have cor pulmonale and right heart failure, with hepatomegaly, ascites, and severe peripheral edema.
Investigations: The chest x-ray, electrocardiogram, and pulmonary function tests are of little value in differentiating thromboembolic pulmonary hypertension from other forms of pulmonary hypertension, though they often give the initial clues that pulmonary hypertension exists.
The most useful screening studies are two-dimensional surface echocardiography with Doppler imaging and radionuclide ventilation/perfusion scanning.
The echocardiogram typically demonstrates right atrial, right ventricular, and pulmonary artery enlargement, with attendant right ventricular hypertrophy. The interventricular septum may be flattened and often will exhibit paradoxical motion, with encroachment of the right ventricular septum into the left ventricle. Varying degrees of tricuspid regurgitation are usually seen. Continuous wave Doppler of the tricuspid regurgitant jet is helpful in estimating the pulmonary artery systolic pressure (PAP) (PAP = [tricuspid envelope]2 × 4 + CVP). Because exercise typically increases the pulmonary hypertension, echocardiography should be repeated with exercise whenever the disease is suspected, but the resting echocardiogram demonstrates only subtle abnormalities.
A perfusion scan should be performed. The major differential diagnosis is from that of primary pulmonary hypertension, where the scan is usually normal, or has a patchy and mottled appearance, in contrast to the multiple punched-out lobar or segmental defects of chronic thromboembolic disease.
Computerized tomography scanning is increasingly being used in the diagnosis of this disease. These images are capable of confirming occlusion of at least the main and lobar pulmonary arteries, but may miss smaller degrees of occlusion. Occlusion localized to the main pulmonary vessels is an unusual finding, and may point to a pulmonary artery tumor. A mosaic pattern of lung attenuation at CT is a sign of variable regional perfusion and will suggest chronic pulmonary thromboembolism.
The pulmonary angiogram remains the gold standard for the diagnosis. Together with right heart catheterization it evaluates the severity of pulmonary hypertension, assesses the surgical accessibility and operative risk, and excludes other diagnoses. Measurement of the pulmonary vascular resistance ([mean PA pressure − mean LA pressure]/cardiac output = PVR in Wood Units; Wood Units × 80 = resistance as dynes/sec/cm−5) is a useful tool. A PVR above 1,000 dynes/sec/cm−5 is a relative risk factor, particularly if associated with only moderate pulmonary vascular occlusion seen on angiogram, though this should not preclude operation.
Both MRI and CT are additive to the angiogram and need not be performed if the angiogram is negative, or clearly diagnostic.
In general, the lower lobes of the lung are more involved with the occlusive process than the upper lobes and the right lung more affected than the left. This may be because of the preferential flow to the right side. Although the term “surgically accessible” thromboembolic disease is often used, pulmonary hypertension as a result of emboli is always operable. The possibility of restoration of completely normal flow to the pulmonary vascular bed, however, may depend on the pattern of thrombotic occlusion and the presence of a secondary vasculopathy.
In addition to pulmonary angiography, patients over 45 years of age undergo coronary arteriography and other cardiac investigation as necessary. If significant disease is found, additional cardiac surgery is performed at the time of pulmonary endarterectomy. Because patency of the distal pulmonary vasculature is almost never a consideration in these patients, selective bronchial artery angiography adds little to the assessment of the patient, and is not indicated.



