Pulmonary Infection in Immunocompromised Hosts
OVERVIEW
A growing group of individuals who have acquired immunodeficiency syndrome (AIDS), who are receiving immunosuppression for solid-organ transplantation, bone marrow transplantation (BMT), or for autoimmune (“connective tissue” diseases), who have primary immune deficiencies, or who have been treated with chemotherapeutic regimens for cancer, have increased susceptibility to infection as a result of acquired or intrinsic immune deficiencies. Prolonged survival of such immunocompromised individuals reflects the deployment of newer laboratory assays and newer antimicrobial agents, including antifungal, antibacterial, and antiviral agents (e.g., ganciclovir, foscarnet, oral agents) and highly active antiretroviral therapies (HAART) for HIV infection, hematopoietic growth factors, and clinical experience in caring for such patients. Wide use of immunosuppressive drugs, including “biologic agents,” which are generally antibodies targeting specific cell types or pathways of inflammation, has further expanded susceptible populations.
Major challenges in providing care to these patients include systemic infections for which therapies or vaccines are limited or absent (e.g., management of respiratory viruses) and progressive antimicrobial resistance of common pulmonary pathogens, including Staphylococcus aureus, Enterococcus, Pseudomonas, Acinetobacter, Stenotrophomonas (formerly Xanthomonas), and Burkholderia species. In addition, selection of novel strains of bacteria, including nontuberculous mycobacteria and Nocardia species, has proved challenging. Resistance has increased to antimicrobial agents commonly used for prophylaxis (i.e., TMP-SMX and fluoroquinolones).
An essential difference exists in the management of pneumonia in immunocompromised individuals when compared with normal hosts. Given the broad spectrum of potential pathogens and of noninfectious processes that may mimic infection, coupled with the inherent toxicities of many therapies, a specific microbiologic diagnosis should be considered essential to the management of pulmonary processes in the immunocompromised host.
GENERAL PRINCIPLES OF MANAGEMENT OF OPPORTUNISTIC INFECTION
Important principles underlying the management of opportunistic infections in immunocompromised patients are discussed below.
Opportunistic infection is defined as infection occurring as a result of compromised immune function and that would not be expected to occur, or would otherwise cause disease of lesser intensity in the presence of normal immune function. Thus, immunocompromised individuals are subject to infections commonly present in the community; however, these infections are likely to be of greater frequency or severity than in the immunologically normal host. In addition, infection in these patients may be caused by organisms of low native virulence or that cause insignificant disease in the normal host, including such organisms as Pneumocystis jiroveci (PCP) or cytomegalovirus (CMV).
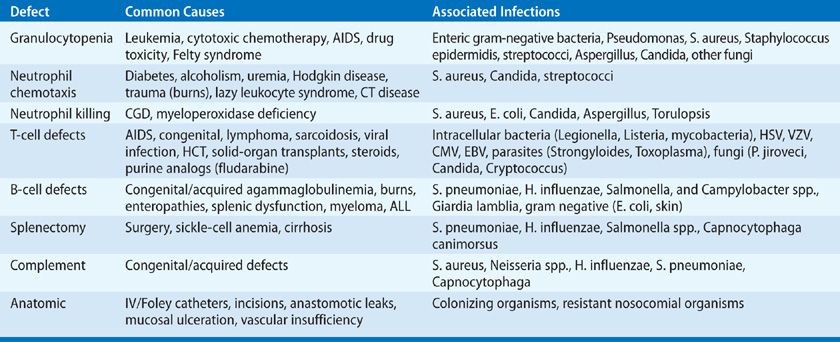
The risk of infection in any patient is determined by the interaction of two factors: the potential pathogens to which the individual is exposed (epidemiologic exposures), and a measure of the individual’s susceptibility to infection, termed the “net state of immunosuppression” (Table 123-1).1–3 The occurrence of infection in an individual at a time when the immune status of the patient is thought to be nearly normal is evidence that either an excessive environmental exposure has occurred or that the immune status of the individual is depressed. Conversely, even minimal environmental exposures may cause invasive infection in an individual who is maximally immunosuppressed.
EPIDEMIOLOGIC EXPOSURES
Epidemiologic exposures of importance to the immunocompromised patient may be divided into three general categories: those occurring within the community, those occurring within the hospital, and those associated with infection transmitted with donor cells or organs (transplantation). Exposures within the community vary, based on such factors as geography and socioeconomic status, and may be recent or remote in time. Thus, opportunistic pathogens acquired in the community include the geographically restricted systemic mycoses (blastomycosis, coccidioidomycosis, and histoplasmosis), Mycobacterium tuberculosis, Strongyloides stercoralis, Leishmania donovani, P. jiroveci, Legionella species, and community-acquired respiratory viral infections (e.g., influenza, adenovirus [AV], respiratory syncytial virus [RSV], parainfluenza virus [PIV], and metapneumovirus). Common viral agents may include herpes simplex virus (HSV), CMV, varicella-zoster virus [VZV], and hepatitis B and C viruses. Due to the limited effectiveness of many vaccines in immunocompromised individuals, infections due to influenza, Streptococcus pneumoniae, and Haemophilus influenzae are common.
Within the hospital, excessive environmental exposures may be divided into two general categories: domiciliary and nondomiciliary. Domiciliary exposures occur on the hospital unit where the patient is housed. When the air, food, equipment, or potable water supply is contaminated with pathogens such as Aspergillus species, Legionella species, or vancomycin-resistant enterococci (VRE), clustering of cases of infection in time and space will be observed. As a result, an increased incidence of nosocomial pneumonia or catheter and wound infections may be seen. Nondomiciliary exposures occur when the patient is transported to contaminated operating rooms, radiology suites, or catheterization laboratories for procedures. Nondomiciliary outbreaks, although possibly more common, are more difficult to detect because of the lack of clustering on a particular hospital unit. The leading clue to the presence of a nosocomial hazard is the occurrence of opportunistic infection in a patient whose net state of immunosuppression would not normally lead to such an event or nosocomial infection with organisms not known to be present on the clinical unit on which the patient is housed.
 NET STATE OF IMMUNOSUPPRESSION
NET STATE OF IMMUNOSUPPRESSION
The “net state of immunosuppression” (Table 123-1) is a conceptual framework for the host factors that contribute to infectious risk.1 These include: the dose, duration, and temporal sequence in which immunosuppressive drugs are deployed; injuries to the primary mucocutaneous barrier to infection (e.g., indwelling catheters and drains); leaks and fluid collections (hematoma, effusions, ascites); ischemic or underventilated tissues; surgical anastomoses; neutropenia or lymphopenia; underlying immune deficiency; pulmonary aspiration injury; metabolic problems including protein–calorie malnutrition, uremia, and hyperglycemia; and infection with immunomodulating viruses (CMV, Epstein–Barr virus or EBV, hepatitis B or HBV, and hepatitis C or HCV, influenza, and the human immunodeficiency viruses, HIV). These viruses predispose to other opportunistic infections and also to graft rejection and to graft-versus-host disease (GVHD). Foreign materials (e.g., vascular grafts, heart valves, sutures) provide a nidus for infection for an organism that would not be capable of causing infection under normal conditions. Thus, Salmonella infection in the organ transplant recipient “homes” to vascular anastomoses or grafts and may persist despite appropriate therapy, causing mycotic aneurysms.
The sum of the congenital, metabolic, operative, and surgery-related factors is the patient’s “net state of immune suppression.” Generally, more than one factor is present in each host; the identification of the relevant factors, and correction when possible, is central to the prevention and treatment of infection in these hosts. For example, in the lung transplant recipient with CMV infection and thoracic fluid collections, the net state of immune suppression includes the immunosuppression used to maintain graft function; diminished immunologic responsiveness due to CMV; the risk of empyema and bacteremia from fluid collections at surgical sites; exposure to, and colonization with, community-acquired and nosocomial organisms; and new infections (e.g., aspiration, Clostridium difficile colitis) that may occur during the prolonged waiting period for a compatible organ. When the organ for transplantation becomes available, it may carry latent infection (e.g., CMV, EBV) or organisms acquired by the donor during hospitalization (e.g., bacteremia) or procurement. The recipient is often critically ill and is subjected to a major surgical procedure. After surgery, the lungs are apt to be compromised, recovery of function in the allograft is often slow, immunosuppressive drugs are initiated, intravenous and urinary catheters and drains are placed, and major incisions need to heal. Drug toxicities are common and may result in renal dysfunction, confusion, or neutropenia. The sum of the underlying, operative, and transplant-related factors is the net state of immune suppression.
Pulmonary defense mechanisms are compromised to varying degrees in immunocompromised hosts (see Chapter 121). Structural defects (fibrosis, airway or lymphatic obstruction by tumor, emphysema) interfere with normal clearance mechanisms. Clearance mechanisms are further impaired by excess mucus and ciliary defects, as in cystic fibrosis, congenital defects, and postlung transplantation, including those due to recent viral or bacterial infections. For example, Mycoplasma shears off epithelial cilia, incapacitating the mucociliary elevator. Viral infections may immobilize pulmonary macrophages (CMV), produce local or systemic immunosuppression, and disrupt the local cytokine network. Alveolar macrophages are diminished in number and function in neutropenic hosts and in stem cell transplant (SCT) recipients. These effects are amplified in the lung transplant recipient in whom the impact of systemic immunosuppression is added to the possible effects of tracheal anastomotic narrowing and tissue ischemia, an impaired cough reflex, progressive bronchiolitis obliterans syndrome, decreased pulmonary T-cell and macrophage functions, hypogammaglobulinemia, and disrupted lymphatic drainage. Thus, in the solid-organ transplantation recipient, the immunosuppression used to prevent graft rejection, as well as technical factors, the diminished host immune responses in the major histocompatibility-mismatched organ, and the impact of immunosuppressive viral infections are the main mediators of susceptibility to infection.
In the stem cell recipient, the intensity of immunosuppression varies with the degree of histocompatibility mismatch between donor and recipient. Nonmyeloablative regimens have reduced the duration of the initial period of neutropenia. However, susceptibility to infection in both autologous and allogeneic HCT recipients persists despite immune reconstitution. Innate immune function is reduced early (first 6 months) as NK cells and alveolar macrophages suffer dysregulation of cytokine, eicosanoid (leukotrienes, prostanoids), and intracellular signaling, and alterations in scavenger receptors, resulting in reduced phagocytosis and bacterial and fungal killing. Over time, persistent reductions are observed in multiple T- and B-cell lineages and maintained in the setting of chronic GVHD. T-cell functions are suppressed by the immunosuppressive agents used to prevent or treat GVHD.
TIME LINES OF INFECTION
With standardized immunosuppressive and chemotherapeutic regimens, specific types of infections often occur in a predictable pattern (“time line”) as a reflection of the specific risk factors (Figs. 123-1 and 123-2) present at each phase of the posttransplantation course. These time lines are altered by specific immunosuppressive regimens, use of antimicrobial prophylaxis, and individual factors (e.g., underlying immune deficits, microbial colonization, organ dysfunction, epidemiologic exposures), but they are useful in considering the “likely” etiology of infectious syndromes in immunocompromised hosts.
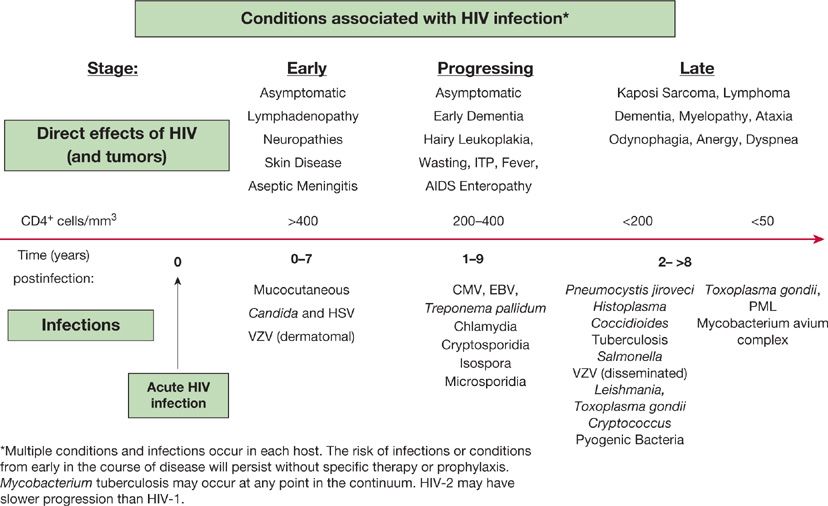
Figure 123-1 The progression of AIDS-associated conditions without effective antiretroviral therapy.
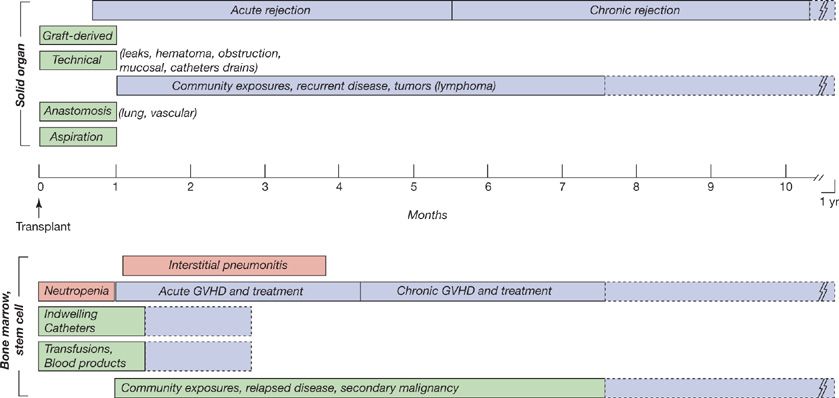
Figure 123-2 The time lines of conditions predisposing to infection in solid-organ transplantation (above the time line) and in bone marrow and stem cell transplantation (below the time line). Patients vary in individual susceptibility patterns and with regard to prophylactic therapies.
The time lines (Figs. 123-1 and 123-2) are used in a number of ways: (1) To develop a differential diagnosis for infectious syndromes by time posttransplant—what type of infections are most likely at various times after transplantation. (2) To develop prophylactic strategies for each patient population based on general considerations (e.g., duration of neutropenia or of T-cell defects) and patient- or institution-specific patterns (e.g., microbial resistance, drug allergies). (3) To identify excess epidemiologic hazards, including nosocomial hazards, for example, Aspergillus, MRSA, VRE, which may be clustered in time and space, by inpatient unit, by procedures, or surgical suite; community exposures, for example, respiratory viruses, Legionella, other outbreaks (SARS); and individual risks, including unique exposures (e.g., hobbies), travel, and occupational hazards. (4) To assess excessive immunosuppression—too many infections or unusual severity and/or at the wrong time on time line suggesting that a problem exists with routine immunosuppressive regimens.
The patterns are altered by antimicrobial prophylaxis, by antiviral therapies for HIV, CMV, and hepatitis B and C viruses, the broader range of immunosuppressive and chemotherapeutic agents, and the use of nonmyeloablative conditioning regimens for HCT. However, the general concepts and major determinants of infection remain the same—the epidemiology of the patient and the intensity of immunosuppression coupled with mucosal injuries and other individual risk factors. Superimposed viral infections will increase the risk of opportunistic infection at any point along the time line.
In immunocompromised hosts, the risks of infection may be relatively stable over time, as in the diabetic with vasculopathy and neuropathy who is prone to skin and soft tissue infections. The risks of infection may be time-limited, as in the postsurgical patient without complications. The risk of infection may be cumulative and progressive, as in the untreated patient with AIDS, in whom infection is a function of declining immune function and immune memory, falling CD4– lymphocyte counts, rising viral loads, and the effects of persistent infections (CMV, Cryptosporidium) (Fig. 123-1). In these individuals, the occurrence of new types of infection suggests the progression of immune compromise.
The risks of infection may also change predictably with time as a function of relatively standardized immunosuppressive regimens and the evolving condition of the patient, as in allogeneic HCT or solid-organ transplantation. For example (Fig. 123-2), in the early phase after bone marrow or hematopoietic cell transplantation or the “pre-engraftment phase,” the risk for infection is driven by neutropenia and mucositis and is generally related to nosocomial or endogenous exposures (gram-negative and gram-positive bacteria, Candida and Aspergillus species) during neutropenia. With nonmyeloablative preparative regimens, this period is generally 14 to 21 days, but it may be significantly longer. This reflects microbial colonization, pre-existing infections, and the impact of vascular access catheters, as well as defects in innate immune function and in mucosal barriers. Subsequently, following marrow engraftment (“early postengraftment phase” of 3 weeks to 3–6 months), but with continued immunosuppression and the absence of full cellular immune function, viral infections predominate, including pneumonitis or colitis (HSV, shingles, CMV, respiratory and enteric viruses), as well as encapsulated bacteria, Pneumocystis, and Aspergillus. In this period, unusual infections (toxoplasmosis) and endemic pathogens must also be considered. Thus, “common” pathogens might be dengue virus and tuberculosis in Southern Asia, or paracoccidioides and tuberculosis in Brazil. Full immune recovery is not achieved before 12 to 24 months, and gaps in immune function may persist for longer periods. During the development of, and treatment for, acute and chronic GVHD, susceptibility to infection is a function of the intensity of immunosuppression and mucosal injuries (from GVHD, chemotherapy, radiation, or infections such as C. difficile colitis). In patients with GVHD in particular, bronchiolitis obliterans, posttransplant lymphoproliferative disorders, and idiopathic pneumonitis syndrome may be observed. Nonmyeloablative conditioning has reduced the rate of diffuse alveolar hemorrhage (early), hepatic veno-occlusive disease (HVOD), and idiopathic pneumonitis syndrome.
In the solid-organ transplant recipient, immunosuppression is used for the life of the transplanted organ. Early (first month) nosocomial infections (surgical and nosocomial) and donor-derived infections are observed but opportunistic infections are not generally observed as the full impact of immunosuppression is not yet appreciated. After a month, as the effects of immunosuppression are maximized, viral infections (CMV, EBV, other herpesviruses, BK polyomavirus, respiratory viruses) predominate, and opportunistic pathogens (Nocardia, Toxoplasma, Cryptococcus, Pneumocystis, mycobacteria, and endemic species) are observed. The endemic species include community-acquired infections, such as geographically restricted fungi (Histoplasma, Coccidioides, Blastomycosis, Paracoccidioides) and parasites (Chagas’, Leishmania).
Because each risk factor renders the patient susceptible to infection by new groups of pathogens, infections occurring with the “wrong” pathogen or at the wrong time suggest an undiscovered “immune deficit” (fluid collection, neutropenia) or an unusual epidemiologic exposure (Table 123-2). The occurrence of specific infections can be prevented by the use of antimicrobial prophylaxis, vaccines, and behavioral modifications (e.g., no raw vegetables or digging in gardens without masks). This will result in a “shift to the right” of the time line in that infections are delayed but incompletely prevented unless the intensity of immunosuppression is reduced.
MICROBIAL VIRULENCE
The risk of infection in any individual patient depends not only on the sum of the immune deficits and epidemiologic exposures, but also on the virulence of the organism relative to pulmonary defense mechanisms. Innate and adaptive immune responses will be blunted by immunosuppression, but also, when activated by infection, contribute to rejection of lung allografts or to GVHD. Such factors as the distribution of toll-like receptors (TLRs) and other pattern recognition receptors (PRRs), microbial production of biofilm, and antimicrobial resistance patterns will influence the pathogenesis of infection. Host cells may enhance the virulence of the invading organism by the induction of genes in that organism that contribute to bacterial persistence or invasion. Thus, resistance to phagocytosis is induced by target cells in Yersinia infections.
Another example of the host–pathogen interaction is the role of CMV in transplantation. CMV is the cause of common clinical syndromes in immunocompromised patients. Among these are fever and neutropenia, pneumonitis, hepatitis, glomerulonephritis, gastritis, colitis, retinitis, and mononucleosis-like syndromes. CMV also induces an array of host responses (i.e., immune suppression, upregulation of histocompatibility antigens and other cell surface antigens, tumor necrosis factor alpha (TNFα) secretion, diminished antigen presentation, graft rejection) that contribute to the host’s susceptibility to infection.4 This is the result of “viral parasitism”—alterations in the target cell including decreased mobility, phagocytosis, and apoptosis, to assure the survival of the virus. Thus, the concept of “immune status” and “epidemiologic exposure” may require modification based on the virulence and immunoregulatory effects of some pathogens.
The incidence of antimicrobial resistance among bacteria, fungi, and viruses is amplified in the immunocompromised host due to repeated exposures to antimicrobial therapies (often for excessive periods at inadequate doses) and repeated hospitalizations. Increasingly, Streptococcus and other gram-positive species are detected with resistance to penicillins, fluoroquinolones, and macrolides. Enterococci are resistant to β-lactam antimicrobials, macrolides, vancomycin, teicoplanin, linezolid, quinupristin- dalfopristin, and aminoglycosides. Pseudomonas, Stenotrophomonas, and the enteric gram-negative bacteria are often resistant to fluoroquinolones and the broad-spectrum carbapenems. Candida and Aspergillus species may be resistant to the azole antifungals.
PRETREATMENT EVALUATION AND PROPHYLAXIS
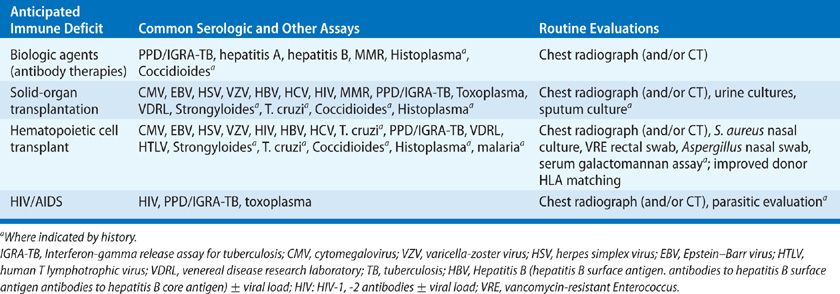
Guidelines for the prevention of infection in common immunodeficiency states are available for HCT, solid-organ transplantation, and AIDS.5–10 The clinical evaluation of the patient prior to immunosuppression may be very helpful in preventing disease (Table 123-3). This evaluation should include a careful epidemiologic history (travel, immigration, exposures including tuberculosis, HIV, endemic fungi and S. stercoralis), a vaccination history including Pneumococcus and H. influenza, varicella zoster, measles, mumps and rubella, diphtheria, pertussis and tetanus, and hepatitis B, exposure to Bacillus Calmette-Guerin (BCG), and chest radiography. Testing for tuberculosis (skin test or interferon-gamma release assay, IGRA) should be routine. Careful evaluation (baseline chest computed tomography [CT] scans) and pretreatment of PPD-positive patients and patients from areas endemic for tuberculosis are advised before HCT and common in solid-organ recipients. Live virus vaccinations should be avoided in immunocompromised hosts and are best provided well in advance of immunosuppression. Pretransplant cultures may guide prophylaxis and establish the presence of antimicrobial susceptibility patterns in advance of invasive infection. Serologic studies are often helpful in the stratification of risk for infection in the immunocompromised host (see Table 123-3). The use of CMV seronegative blood products in seronegative individuals may avoid acute infection during periods of intensive immunosuppression.11
Given the routine use of antimicrobial prophylaxis in the face of persistent immune defects, infection remains common due to organisms that are resistant to the agents employed. Prophylaxis falls into three categories: (1) Primary or universal prophylaxis in which all at-risk patients receive an agent to prevent common infections (TMP–SMX for Pneumocystis). (2) Secondary prophylaxis initiated after treatment to maintain remission or prevent recurrence. (3) Preemptive therapy in which a sensitive diagnostic assay is used for screening with treatment initiated based on the presence of a positive assay to avoid progression of disease. Oral agents including TMP-SMX, fluoroquinolones, acyclovir (and related agents), and azole antifungal drugs have widespread use in the management of immunosuppressed hosts. Oral decontamination regimens (i.e., nonabsorbable antimicrobials) have not been proven to prevent disease beyond limited periods of time, and are poorly tolerated because of taste, consistency, malabsorption of glucose and xylose bases, and cost.
INITIAL MANAGEMENT OF THE IMMUNOCOMPROMISED HOST WITH INFECTIOUS SYNDROMES
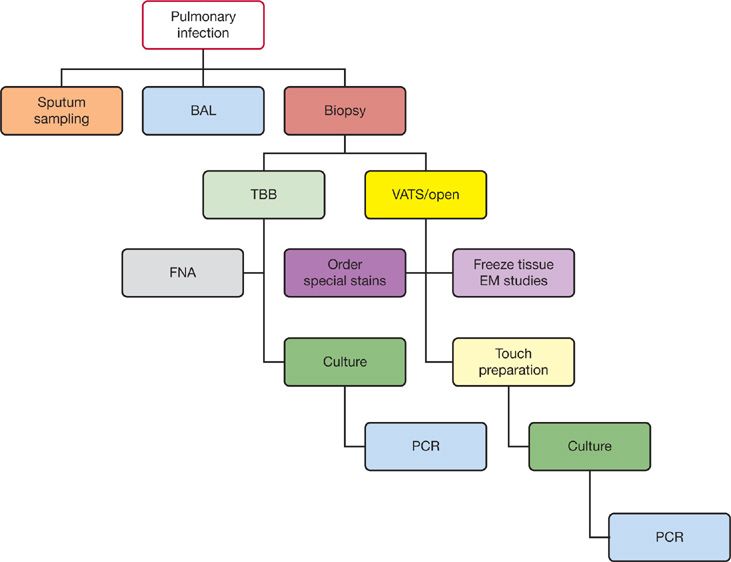
Infections in immunocompromised hosts often present without the expected signs and symptoms of infection. The presence of nonspecific clinical manifestations may delay identification of the critically ill patient. In the outpatient setting, the practitioner must have a low threshold for performing diagnostic tests (e.g., blood counts, cultures, radiographs) on patients with minimal symptoms. Appropriate cultures and invasive studies such as bronchoscopy or biopsy are best performed early in the course of possible infection and prior to the initiation of antimicrobial therapy.12 The handling of clinical samples is outlined in Table 123-4 and in Figure 123-3. In practice, most febrile or possibly infected immunocompromised patients are treated empirically while awaiting data that identify specific pathogens. The selection of empiric therapy depends on the nature and intensity of the patient’s immune deficit(s), risk for poor outcome (severe neutropenia, sepsis), the likely sites of infection, antimicrobial susceptibility patterns at the institution, and the toxicities and cost of the appropriate regimen. Synergistic antimicrobial therapy for known or suspected pathogens must be used when available. Agents or classes of agents used for prophylaxis should not be used for therapy of possible break-through infection. Compromises are often made; loss of renal function will significantly hinder patient management. Thus, a balance is often made between optimal empiric therapy (e.g., empiric broad-spectrum antifungal therapy with lipid amphotericin vs. other agents) and other aspects of patient management. However, progression of infection while on static or inadequately dosed antimicrobial agents must be avoided.
TABLE 123-4 Routine Laboratory Evaluation of Bronchoalveolar Lavage Specimens in Immunocompromised Hosts
Figure 123-3 The diagnostic workup of pulmonary infection.
Clinical signs predicting poor outcomes mitigate to broader initial therapy (e.g., including antifungal or antiviral agents) with adjustment based on microbiologic data at 24 to 72 hours and after the patient is stabilized. These worrisome signs include hypotension, newly altered mental status, meningitis, or encephalitis, diffuse or necrotic skin lesions, bleeding diathesis or new thrombocytopenia, vascular thrombosis, hypoxemia, typhlitis or acute abdomen, severe hyperglycemia, pancreatitis, or lactic acidosis, hypercalcemia, perirectal abscess, gas gangrene, or Fournier gangrene. The radiologic appearance of pneumonia is altered by immune suppression (Fig. 123-4). Radiographic patterns may also change during the care of the patient (e.g., cavitation of pulmonary nodules after the resolution of neutropenia).
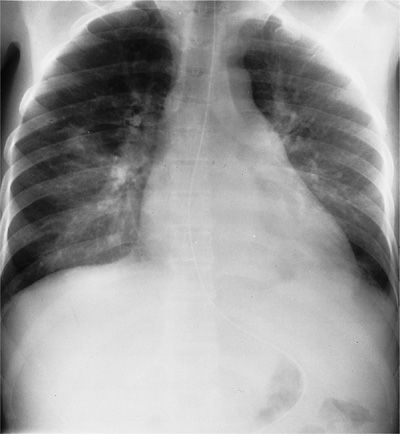
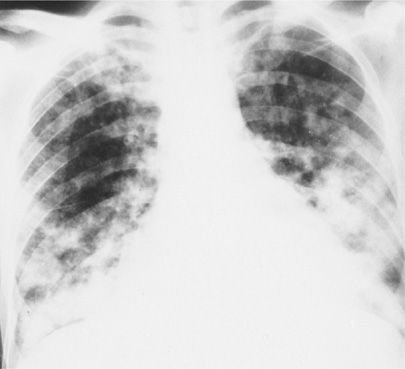
Figure 123-4 Chest radiograph of a 48-year-old heterosexual man with community-acquired pneumonia unresponsive to therapy. The patient was diagnosed as having AIDS on the basis of HIV seropositivity, CD4+ lymphocyte count of 113 per milliliter, and P. jiroveci and Mycobacterium avium-intracellulare complex pneumonia.
Antimicrobials alone may not suffice in the treatment of infection in the immunocompromised host.9 Infections may respond to a decrease in exogenous immune suppression, to correction of neutropenia by growth factors, or to treatment of simultaneous infections that predispose to superinfection (e.g., RSV, CMV). It should be noted that cessation of corticosteroids may provoke adrenal corticosteroid insufficiency with associated hypotension and metabolic complications. Similarly, cessation of immunosuppression may provoke GVHD or graft rejection or flares in other underlying diseases, and immune reconstitution syndromes will unnecessarily complicate the management of pneumonia and other processes.
Drainage of collections of infected fluid such as empyemas, hematomas, or lymphoceles, or removal of drains or catheters will enhance the clinical response. Identification of metastatic sites of infection (e.g., infections spreading from the lungs to the central nervous system due to Nocardia, Aspergillus, or Cryptococcus species) may facilitate diagnosis and management. The identification of new infectious disease syndromes has occurred in individuals with immune deficits. Thus, the cluster of cases of P. jiroveci pneumonia in homosexual males was the first indicator of a new viral pathogen (HIV-1), and the role of Cryptosporidium as a common cause of diarrhea in both normal and compromised individuals was elucidated as a result of diarrheal disease in AIDS patients in the 1980s. Similarly, many uncommon bacteria (Bartonella species, Rhodococcus equi), viruses (Kaposi sarcoma-associated herpesvirus/human herpesvirus 8, polyomaviruses, SARS coronavirus), fungi (Penicillium, Scedosporium), and parasites (Microsporidia) have been identified in immunocompromised patients. Thus, continuing consideration of new pathogens or novel presentations of known pathogens is essential for the care of the immunocompromised patient.13
 RECOGNITION OF CONCOMITANT NONINFECTIOUS PROCESSES
RECOGNITION OF CONCOMITANT NONINFECTIOUS PROCESSES
The occurrence of multiple simultaneous infections or conditions often complicate and delay appropriate therapy (Fig. 123-4). For example, CMV infection may complicate the treatment of graft rejection or GVHD and contribute to the pathogenesis of Pneumocystis or Toxoplasma pneumonia. In the compromised host with fever and pneumonitis, chest radiographs may be difficult to interpret. Noninfectious causes of pulmonary infiltrates may coexist with infection, and atypical patterns predominate. Drug toxicities (bleomycin, cyclophosphamide, sulfa drugs), leukoagglutinin reactions, engraftment or immune reconstitution syndromes, radiation injury, pulmonary emboli, and lesions of metastatic cancer may coexist with opportunistic infection (Fig. 123-5). The “typical” evolution of pulmonary infection may be altered by the presence of underlying (e.g., interstitial) pulmonary disease, as well as by diminished inflammatory responses. It is commonly necessary to repeat tests, to utilize CT, or to use invasive diagnostic modalities (biopsy) in the evaluation of the patient who is unresponsive to therapy. Complications of therapy may contribute to the development of new infections: TMP-SMX can cause pneumonitis, hepatitis, or Stevens–Johnson syndrome; ganciclovir can cause neutropenia; transfusion reactions can cause pulmonary infiltrates and hemolysis; cyclosporine can cause hemolytic-uremic syndrome; and antimicrobials can contribute to thrush and C. difficile colitis.
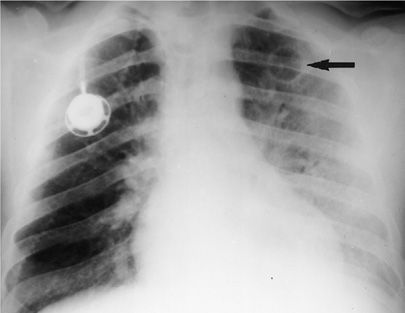
Figure 123-5 Chest radiograph of a 36-year-old homosexual man not known to be HIV-1 infected, with bilateral nodular infiltrates due to pulmonary Kaposi sarcoma.
HIV INFECTION AND AIDS
The spectrum of common infections varies with specific immune defects in each type of host (Table 123-2), including HIV infection.
The management of HIV infection has been dramatically altered for those individuals with access to combinations of drugs in “highly active antiretroviral therapies” or HAART.14,15 Prior to therapy, there is marked variability in the pattern of disease progression with viral load reaching a near steady state by approximately 6 months after infection. Rapid loss of CD4+ lymphocytes correlates with HIV viral load and precedes waning humoral immunity.16–23 Of note, B cells demonstrate activation and proliferation with nonspecific polyclonal hypergammaglobulinemia. Thus, susceptibility to bacterial infections and recurrent pneumonia rises with deficiency in the production of specific antibodies. The degree of immunodeficiency correlates with loss of CD4+ cells with untreated progression to AIDS occurring within 8 to 10 years; levels of less than 50/mm3 have a median survival of less than 2 years.
Underlying lung disease is common in HIV-infected patients even before the development of opportunistic infection. While FEV1 and FVC are nearly normal, 11% to 13% of patients with CD4+ lymphocyte counts below 200/mm3 or with a history of AIDS-associated extrapulmonary diseases (including thrush and varicella-zoster infections) and weight loss have decreased DLCO measurements. Intravenous drug users have a higher incidence of abnormal FVC, FEV1, and DLCO measurements (33.3%), consistent with patterns of cigarette smoking and racial distribution. Thus, susceptibility to pulmonary infection is further exacerbated in this population.
One of the features of HAART is a syndrome of inflammatory responses referred to as the “immune reconstitution syndrome,” which generally occurs within the first 3 months of starting effective antiretroviral therapy. This is thought to represent a hyperacute response to pathogens to which the HIV-infected individual has been exposed. It has been observed in P. jiroveci pneumonia, CMV retinitis and vitritis, disseminated Mycobacterium avium complex (MAC), tuberculosis, and histoplasmosis as pneumonitis and lymphadenitis, cryptococcosis with meningitis, hydrocephalus, and necrotizing lymphadenitis, and with acceleration of hepatitis C virus infection including cryoglobulinemia and renal failure. Thus, effective antiviral therapy may result in more intense symptoms and unusual manifestations of some opportunistic infections while the overall incidence of new infections has declined.
For adults and adolescents (i.e., persons aged ≥13 years), the surveillance case definitions for HIV infection and AIDS were revised into a single case definition for HIV infection in 2008 that includes AIDS and incorporates HIV infection staging classification system.24 A confirmed case meets the laboratory criteria for diagnosis of HIV infection and one of the four HIV infection stages (stage 1, stage 2, stage 3, or stage unknown).
• HIV infection, stage 1: No AIDS-defining condition and either CD4+ T-lymphocyte count of ≥ 500 cells/μL or CD4+ T-lymphocyte percentage of total lymphocytes of ≥29.
• HIV infection, stage 2: No AIDS-defining condition and either CD4+ T-lymphocyte count of 200 to 499 cells/μL or CD4+ T-lymphocyte percentage of total lymphocytes of 14 to 28.
• HIV infection, stage 3 (AIDS): CD4+ T-lymphocyte count of <200 cells/μL or CD4+ T-lymphocyte percentage of total lymphocytes of <14, or documentation of an AIDS-defining condition. Documentation of an AIDS-defining condition supersedes a CD4+ T-lymphocyte count of ≥200 cells/μL and a CD4+ T-lymphocyte percentage of total lymphocytes of ≥14.
• HIV infection, stage unknown: No information available on CD4+ T-lymphocyte count or percentage and no information available on AIDS-defining conditions.
Anti-HIV therapy should be started before the immune system is irrevocably compromised. Most practitioners are treating all individuals with progressive HIV infection, and all HIV-infected individuals with CD4 counts below 200/mm3. HAART has resulted in the recrudescence of immunity as manifested by rising CD4+ lymphocyte counts and diminished signs of opportunistic infection and cancer associated with severe T-cell deficits. In treated individuals, this has reduced the incidence of AIDS, AIDS-defining diagnoses, hospitalizations, and mortality by 60% to 80%. Treatment of HIV infection appears to virtually eliminate the risk of Pneumocystis pneumonia in AIDS patients with and without prior Pneumocystis pneumonia. The risk of MAC, tuberculosis, and CMV has also decreased. Thus, prophylactic (both primary and secondary) and therapeutic regimens must be considered in light of the individual’s immune status. Not all patients respond to HAART or maintain viral suppression during therapy. The specifics of antiviral therapy will not be considered here.
 HIV TESTING
HIV TESTING
HIV testing should be considered for all persons either in high-risk groups or with unusual infections (Table 123-5). High-risk groups include intravenous drug users, sexually active homosexual or bisexual men, hemophiliacs or individuals who have required blood or clotting factors, persons with sexually transmitted diseases (especially syphilis), people with heterosexual contacts with one of these increased risk groups, pregnant women or newborns with such contacts, healthcare workers with exposure to body fluids or needle stick injury, and all patients with conditions commonly associated with AIDS.
Testing for HIV infection is generally divided into viral culture assays (uncommon now that molecular resistance tests are available), antibody tests (Western blots), and specific, quantitative (molecular) viral tests including molecular antiviral susceptibility testing. Most patients produce antibodies to HIV within 6 to 8 weeks, and almost 100% will have detectable antibodies by 6 months after exposure. These tests are well standardized and easy to perform, but are troubled by false positives (cross-reacting antibodies) and false negatives (e.g., in the early or “window” period). Between 4% and 20% of Western blot tests are indeterminate because of seroconversion in progress, loss of antibody in advanced HIV disease, cross-reacting antibodies in pregnancy, blood transfusions, autoantibodies from collagen vascular disease, infection with HIV-2, recent influenza vaccination, or in recipients of trial HIV vaccines. These subjects should be retested and inconclusive assays resolved with specific viral (molecular, p24 antigen, or culture) testing. Specific viral tests include the p24 antigen detection, molecular amplification by PCR, and culture-based assays. These are positive earlier than the antibody tests and therefore may be useful in primary infection before the development of antibody; they have high sensitivity (95%–99%). Quantitative techniques are very useful in assessing the response to antiviral therapy and disease progression.
Measures of HIV viral RNA in plasma may not correlate with the CD4+ lymphocyte count. The CD4 count provides a surrogate marker for the response to antiviral therapy and the risk of opportunistic infection and death. At present, the best predictive value of testing is the combination of viral load with CD4+ lymphocyte enumeration. Viral RNA levels in long-term nonprogressors are consistently under 10,000 copies/mL, while progression and immunologic deterioration are often associated with loads over 50,000 to 100,000 copies. Patients with viral loads of 10,000 to 50,000 are considered at intermediate risk. Viral load changes generally precede CD4 count changes. Immune alterations due to infection (e.g., CMV) or immune modulation therapy (interferons) are not yet interpretable.
 IMMUNIZATION
IMMUNIZATION
Immunization is a part of the routine management of AIDS. In general, HIV-infected persons are susceptible to the same community-acquired respiratory pathogens as the normal host but with a greater severity of disease. Thus, patients should be vaccinated early in the course of disease when they are clinically stable. Live vaccines are generally contraindicated, but measles vaccine is generally well tolerated in children, and MMR is recommended for unvaccinated adults born after 1957 or vaccinated between 1963 and 1967. The efficacy of vaccination in this population is not clear; HIV viral loads may temporarily increase after vaccination. However, general practice suggests that pneumococcal, influenza (inactivated whole virus and split virus vaccines), H. influenzae, hepatitis B recombinant vaccine, and MMR be given as indicated.
 OPPORTUNISTIC INFECTIONS IN AIDS
OPPORTUNISTIC INFECTIONS IN AIDS
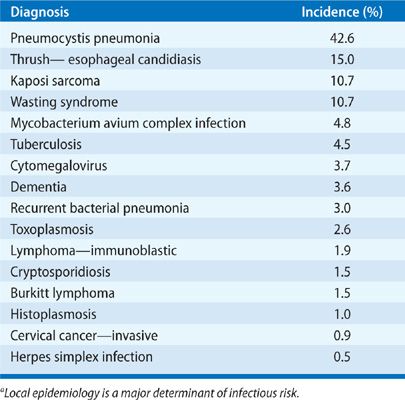
The problem of opportunistic infection in the untreated or newly diagnosed AIDS patient is unique because of the progressive decline in immune function when compared with the intermittent compromise seen after chemotherapy or the relatively stable immunosuppression utilized after solid-organ transplantation (Table 123-6). As a result of the progressive and cumulative risks, the incidence of opportunistic infections increases over time. A “time line” exists for the common infections and noninfectious manifestations seen in progressive AIDS, relating to the total CD4+ lymphocyte count as a measure of susceptibility (Fig. 123-1). In an individual, the time line is also related to the patient’s viral load, but an exact correlation does not exist. The specific pattern of opportunistic syndromes will change for individual patients, but it reflects the overall progressive immunological deterioration of untreated AIDS.
Many opportunistic pulmonary infections in AIDS patients were initially assumed to be reactivation of latent infection. However, some of these processes – including P. jiroveci, Toxoplasma gondii, tuberculosis, and histoplasmosis – represent a mix of both new exposures and old disease. Similar observations have been made in terms of the drug susceptibility of mycobacterial isolates in recurrent disease (see Chapter 131) (Fig. 123-6). The clinical manifestations of opportunistic infections in AIDS are altered by prophylactic and therapeutic regimens, adverse drug reactions, and drug interactions. Toxicities of both prophylactic and therapeutic drug regimens (particularly rash, marrow suppression, and hepatic toxicities) are much more frequent in HIV-infected patients and are exacerbated by the simultaneous use of antiviral therapies.
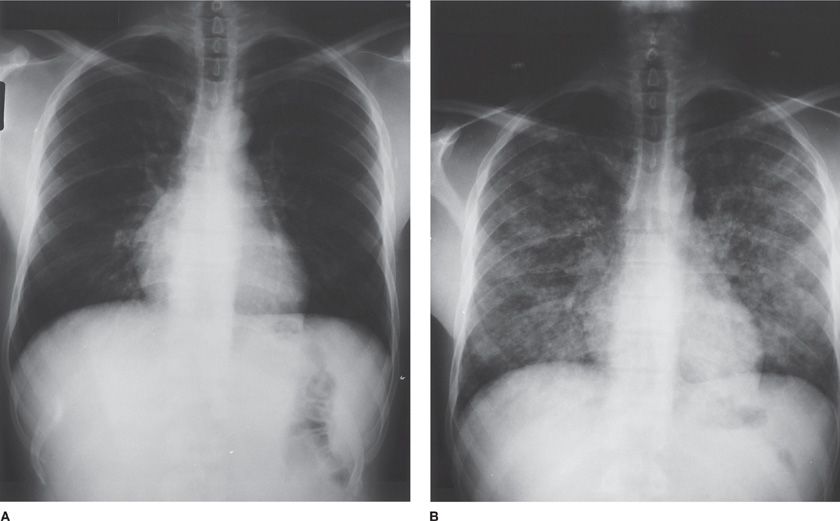
Figure 123-6 Chest radiographs of a 39-year-old man with AIDS on zidovudine, ritonavir, and TMP-SMX prophylaxis, and with a CD4+ lymphocyte count of 89 per milliliter. The patient presented to the outpatient clinic with low-grade fever, fatigue, and mild cough. A. Physical examination and chest radiograph were unremarkable. The patient was anergic on both PPD and control skin testing. Induced sputum examination was negative for bacteria, for P. jiroveci, and by mycobacterial stains. Blood cultures for mycobacteria were obtained. B. Ten days after initial presentation, the patient was admitted to the hospital with minimal dyspnea and cough; chest radiograph was remarkable for bilateral pulmonary reticulonodular infiltrates. Bronchoalveolar lavage samples were positive for mycobacteria. The organisms were subsequently identified from cultures of both blood and sputum as M. tuberculosis, resistant to both isoniazid and ethambutol. Induced sputum sample cultures remained negative for mycobacteria.
Primary prophylaxis in AIDS patients who maintain CD4+ lymphocyte counts above 200/mm3 for over 3 to 6 months and with low or undetectable viral loads appears to be unnecessary, at least for P. jiroveci and mycobacterial infections.25 For individuals with CD4+ lymphocytes below 100/mm3 the incidence of PCP is 40% to 50% and toxoplasmosis 33% per year without prophylaxis. TMP–SMX prophylaxis (a single strength tablet daily) prevents most toxoplasmosis and nocardiosis, listeriosis, salmonellosis, and other common infections.26–29 For MAC, prophylaxis should be considered for HIV-infected individuals with less than 50 to 100/mm3 CD4+ lymphocytes with azithromycin (or clarithromycin).30,31 For other infections and secondary prophylaxis, the data are less clear. Up to 15% to 20% of AIDS patients have more than one opportunistic infection at one time. The spectrum of clinical diagnoses in pulmonary disease in AIDS includes bacterial infection (45.5%), P. jiroveci pneumonia (27%), Kaposi sarcoma (7%), bronchitis (5%), M. tuberculosis (4.3%), other mycobacteria (4%), lymphoma (2.1%), and a variety of other processes. Common community-acquired upper respiratory infections, manageable on an ambulatory basis, constitute more than 50% of respiratory illnesses in HIV-infected persons. The incidence of fungal infections varies by geographic region, while the rate of demonstration of viral pulmonary infection is closely related to the diagnostic testing techniques used at each center and to seasonal variation.
 APPROACH TO THE DIAGNOSIS OF OPPORTUNISTIC PULMONARY INFECTIONS IN AIDS
APPROACH TO THE DIAGNOSIS OF OPPORTUNISTIC PULMONARY INFECTIONS IN AIDS
With the wide array of potential pathogens causing disease in HIV-infected patients, the frequency of atypical and multiple infections, and the urgency to diagnosis of infection in the immunocompromised host, a systematic approach to lung disease in these hosts is imperative. A few general rules are useful.
1. Prophylaxis is generally effective. When failure of prophylaxis occurs, it is usually due to noncompliance, malabsorption of drugs, emerging antimicrobial resistance, or coinfection or tumor that alters the local environment. For example, it is often impossible to eradicate Candida esophagitis unless erosive esophageal HSV infection is also treated. Pneumocystis is difficult to treat in the presence of CMV infection or bronchial obstruction.
2. Specific therapies for individual infections have a high incidence of adverse reactions in the HIV-infected patient. Thus, presumptive or empiric therapy without microbiologic confirmation, though often appropriate, has a greater risk in this population than in the normal host.
3. The utilization of newer diagnostic tests has improved the care of AIDS patients. The interpretation of some tests is unclear, and the availability of some tests (urinary Histoplasma antigen or immunoperoxidase stains for T. gondii) is not universal. The induced sputum examination has been useful in the early, noninvasive diagnosis of Pneumocystis infection, and for mycobacterial disease in the absence of spontaneous sputum production. The sensitivity of sputum induction for Pneumocystis infection approaches 90%, but the negative predictive value of the test is only 50%. The cost and sensitivity of this procedure cannot be justified for the routine diagnosis of bacterial infections, particularly in persons capable of producing sputum samples. The use of more invasive tests, such as bronchoscopy, with the obvious limitations of cost and risk to the patient, has the advantage of providing subglottic specimens and the potential for diagnosis of a broader range of pathogens. The interpretation of positive cultures for CMV or for MAC may be uncertain without tissue histopathology for confirmation. In patients with a rapidly deteriorating clinical condition or a failure to respond to initial therapy, bronchoscopy with biopsy or needle aspiration may be preferable to bronchoalveolar lavage (BAL) or sputum induction as an initial procedure. In general, noninvasive, nuclear isotope-based radiologic tests are rarely useful in the diagnostic evaluation of pulmonary disease in AIDS patients.
4. The rate of progression of infection is often a clue to the type of disease. Thus, community-acquired pneumonia develops rapidly (in 2–5 days), while the initial episode of Pneumocystis pneumonia generally evolves more slowly (over 7–12 days) in AIDS (as compared with other compromised hosts). Fungal infection and mycobacterial infection are generally preceded by systemic complaints. Pyogenic pulmonary infection is generally associated with sputum production, while the “atypical” infections may have little or no sputum despite cough and dyspnea.
5. The radiographic pattern is often suggestive of the diagnosis (Table 123-7). All “typical” patterns are altered by progressive immune deficits and coexisting or prior lung disease. Diffuse infiltrates (alveolar or interstitial) may be seen with a homogeneous distribution, as in P. jiroveci, T. gondii, CMV, mycobacterial species, Histoplasma, or Coccidioides (Fig. 123-7). Drug toxicity may also cause pulmonary infiltrates. Inhomogeneity with these pathogens reflects altered pulmonary parenchyma from previous disease, obstruction (e.g., with tumor, S. stercoralis), or upper zone disease or pneumothorax in Pneumocystis pneumonia (Fig. 123-8). Tumors may appear with interstitial radiographic patterns in HIV disease. Lymphoid interstitial pneumonitis is an interstitial process of unknown origin that is seen in AIDS patients. Diffuse interstitial infiltrates are often due to P. jiroveci, but not in patients receiving TMP–SMX prophylaxis and rarely without hypoxemia. Thus, the presence of a sepsis-like picture with a diffuse interstitial infiltrate in a patient receiving anti-Pneumocystis prophylaxis might suggest mycobacterial disease, Legionella infection, or Cryptococcus neoformans. Focal airspace disease is most often seen with bacterial infections (pyogenic, mycobacteria, Legionella species), Mycoplasma pneumoniae (viral influenza, AV, CMV), and mixed infections (e.g., CMV and P. jiroveci). Occasionally, primary cryptococcal pneumonia, Aspergillus infection, or obstructive disease will present with focal infiltrates. Each of these processes may evolve to frank cavitation, particularly infections due to pyogenic bacteria (Staphylococcus, Klebsiella, S. pneumoniae) or M. tuberculosis. Small cavities are seen with P. jiroveci, mycobacteria, and metastatic tumors. Large cavities are uncommon; M. tuberculosis or aspergilloma is most often present. Nodular lesions can be seen with any of the metastatic tumors or hematogenous infections. Endocarditis, KS, toxoplasmosis, tuberculosis, MAC, and Cryptococcus may all progress from nodules to small cavities (Fig. 123-9). In particular, unusual bacterial pathogens (Bartonella, Rhodococcus, Candida, Salmonella) have been observed as pulmonary nodules associated with right-sided endocarditis in AIDS patients (Fig. 123-10). Intrathoracic adenopathy is common in untreated AIDS patients, most often with infections earlier in the course of disease (CD4+ count greater than 400 per mL) and with tumors later in disease. Fungal infections (Cryptococcus, Histoplasma, Coccidioides), CMV, and mycobacterial infections may also cause adenopathy. Adenopathy should prompt invasive diagnosis in the absence of a clear etiology in AIDS. Pleural effusions are common with tuberculosis, other pyogenic bacterial infections, and tumors.
6. The CD4+ lymphocyte count is a good indicator of susceptibility to specific infections, while the viral load is most closely associated with overall disease prognosis. Unresolving community-acquired pneumonia due to S. pneumoniae, H. influenzae, Mycoplasma, or Legionella species may be the sentinel infection of HIV disease. As host immunity declines, other opportunistic infections will occur. M. tuberculosis, an organism of high virulence, will cause infections at any CD4+ lymphocyte count but will occur increasingly as the CD4+ lymphocyte count falls below 500 per mL. In contrast, less virulent organisms will cause disease only with greater degrees of immune compromise.
7. Chronic or recurrent sinus infection may provide a source of Pseudomonas or Aspergillus for pulmonary infection.
8. The spectrum of pulmonary disease varies by geographic region and by HIV transmission category.
9. Physical findings are often useful in establishing a differential for pulmonary disease in contrast, often, with other types of immunocompromised hosts.
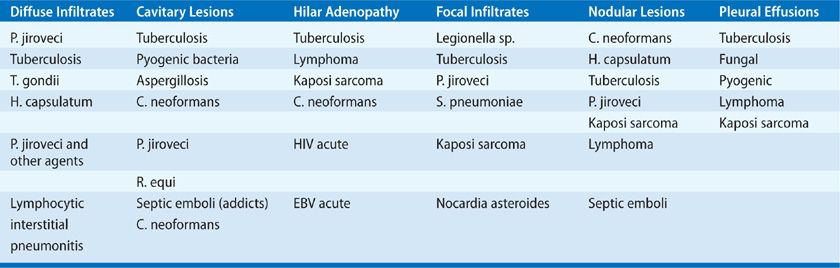
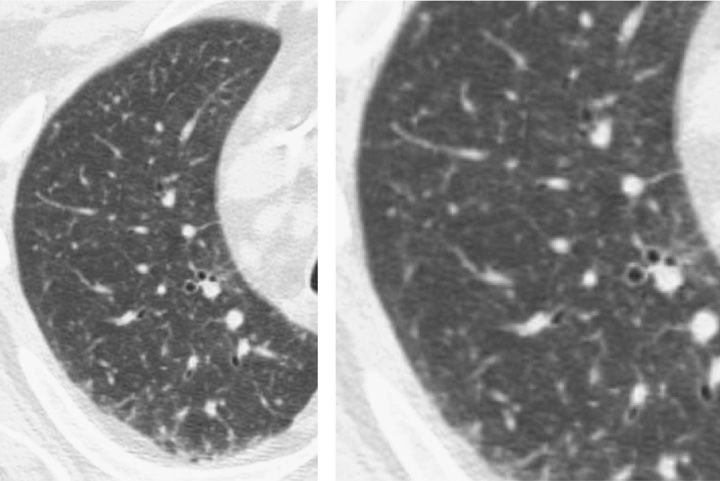
Figure 123-7 Diffuse ground-glass opacity of P. jiroveci pneumonia in AIDS at the time of initial presentation predominantly involved the subpleural lung and spared the central lung.
Figure 123-8 A. A mucus plug in a small airway. B. Higher power view reveals the larval form of Strongyloides stercoralis.
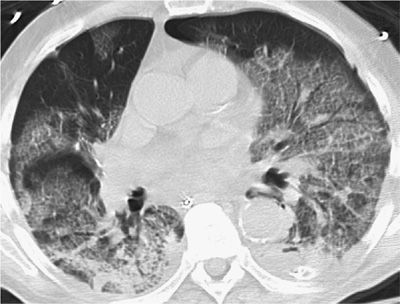
Figure 123-9 Miliary cryptococcal pneumonia in a 62-year-old man undergoing intensive chemotherapy for pancreatic cancer. C. neoformans was isolated from bronchoalveolar lavage specimens and cerebrospinal fluid (CSF). Cryptococcal antigen was positive in serum and CSF at titers greater than 1:1024. CT scan demonstrates diffuse 1- to 3-mm micronodules in both lung fields, often difficult to distinguish from small blood vessels on end. This requires review of serial CT cuts to distinguish nodules from tubular vessel structures.
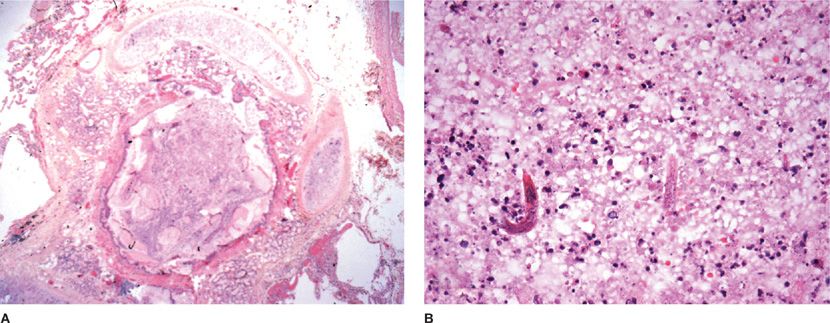
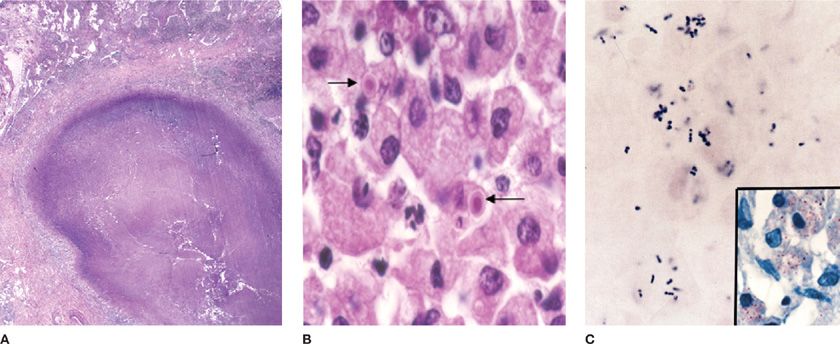
Figure 123-10 A. Rhodococcus equi pneumonia in AIDS produced a cavitary lung nodule in a lung biopsy specimen. B. The host response includes histiocytic inflammation and small calcified concretions termed Michaelis–Gutmann bodies (arrows). C. The organism is a gram-positive coccobacillus that also stains with the modified Ziehl–Neelsen stain (inset).
INFECTION IN PATIENTS WITH CANCER OR HCT
Patients with underlying malignancy who are treated with chemotherapy or HCT represent special challenges with respect to evaluation and management of known or suspected infectious diseases. Important aspects of the underlying basis for their immunocompromised status are discussed below.
 IMMUNE DEFECTS DUE TO TUMORS AND CHEMOTHERAPY
IMMUNE DEFECTS DUE TO TUMORS AND CHEMOTHERAPY
The incidence of infection in cancer patients is determined, in part, by the nature of the underlying neoplasm. Studies of pneumonia in cancer patients suggest the following approximate incidences of infection:
• Conventional bacteria—37%
• Fungi—14%
• Viruses—15%
• P. jiroveci (formerly Pneumocystis carinii)—8%
• Nocardia asteroides—7%
• M. tuberculosis—1%
• Mixed infections—20%
In the series by Bodey et al.,32,33 fatal infections in acute leukemics were caused by bacteria in 66%, fungi in 33%, viruses in 0.2%, and protozoa (including P. jiroveci, now considered a fungus) in 0.1% (Fig. 123-11). In contrast, fatal infection in lymphoma patients (86%) and solid-tumor patients (94%) were more often bacterial in the preprophylaxis era. The rate of cryptococcal infection in chronic lymphocytic leukemia was more than double that in Hodgkin disease (24.3 vs. 10.9 per 1000), and the rate in breast cancer was only 0.159 per 1000.
Figure 123-11 Lung abscess (arrow) in a febrile patient following intensive chemotherapy for relapsed acute myelogenous leukemia. Patient developed fever while granulocytopenic (<50 neutrophils/mm3 for 8 days) without localizing symptoms and a clear chest radiograph. When the neutrophil count exceeded 200/mm3, a lung abscess was detected in the left upper lobe. Aspergillus fumigatus was detected in fluid obtained from the abscess via CT-guided percutaneous needle aspiration. The infection responded well to amphotericin B.
Other tumors are associated with specific infections. For example, lung cancer is associated with tuberculosis at a rate of 92 per 1000, second only to the rate in patients with Hodgkin disease (96 per 1000) due to cellular immune deficits. Without therapy, the degree of depression in cellular immunity (delayed-type hypersensitivity) is more prominent in lymphoma, whereas humoral immunity is impaired to a greater degree in diseases affecting B-lymphocyte function, such as multiple myeloma and chronic lymphocytic lymphoma. Thus, the patient with lymphoma is particularly susceptible to intracellular organisms, including Listeria monocytogenes, M. tuberculosis, viruses, and fungi. The patient with myeloma is more apt to develop pneumonia or bacteremia due to H. influenzae, S. pneumoniae, and a variety of other acute bacterial infections. Acute leukemia is associated with a depression in the number and function of circulating granulocytes and is associated with severe pyogenic, bacterial infections. Patients with acute and relapsed leukemia have demonstrated impaired phagocytosis and killing of fungi and bacteria by these cells, which may appear morphologically normal. These defects may persist well into periods of remission and may progress along with progression of the underlying disease.
The impact of the various forms of chemotherapy on host defenses must be added to those caused by the underlying malignancy. Multiple immune functions are impaired by chemotherapy, including the phagocytosis and killing of bacteria by neutrophils (corticosteroids, carmustine, radiation); antibody production (methotrexate, cyclophosphamide, L-asparaginase, 6-mercaptopurine); uptake and processing of antigen by macrophages (corticosteroids, cyclophosphamide, dactinomycin); recognition of antigens by T and B lymphocytes (corticosteroids, cyclophosphamide); and antigen-driven lymphocyte proliferation (methotrexate, 5-fluorouracil, fludarabine, cytarabine, L-asparaginase, dactinomycin, 6-mercaptopurine, hydroxyurea). Predisposition to infection induced by chemotherapy may mask more subtle defects due to underlying disease; for example, the effects of granulocytopenia due to intensive chemotherapy will generally predominate over the effects of underlying lymphoma or myeloma.
 NEUTROPENIA
NEUTROPENIA
The most common predisposing condition for infection in the patient with cancer is granulocytopenia, often due to chemotherapy and occurring while awaiting engraftment of hematopoietic transplants. The function of inflammatory cells and of other immune (e.g., mucosal) barriers are also of great importance and are much more difficult to assess. The risk of infection increases as granulocyte counts decrease (Table 123-8). Thus, the risk of infection in the patient with neutropenia (under 1000 total granulocytes per mm3) increases when granulocyte numbers fall further, to below 500/mm3; the risk is greatest when counts are lower than 100/mm3.32,33