Epidemiology
Precise epidemiologic data for pulmonary embolism (PE) have been difficult to obtain owing to the relatively high incidence of clinically silent disease and variable rates of autopsy. Nevertheless, it is believed responsible for about 1% of hospital admissions, present in up to 30% of hospital-based autopsies, and considered the cause of the death in ˜3% to 5% of hospitalized patients.
1 Annual clinical incidence rates estimate that venous thrombosis and PE affect 0.5 to 1.0 per 1,000 individuals in a population.
2 The overall death rate among adults is about 20 per 100,000
3 and has been decreasing.
4The rate of PE at autopsy ranges from 15% to 30% and is considered the cause of death in 5% to 12%. The diagnosis is clinically suspected in less than one in five cases, the high incidence possibly due to autopsy selection bias in unexplained sudden death. The rate of PE at autopsy has declined from 20% to 10% over the last several decades.
1,5 The source of emboli includes the lower extremity (70.8%), pelvic veins (4.2%), upper extremity (1.1%), and no source identified (22.6%). The presence of chronic obstructive lung disease has been associated with unidentifiable venous source for pulmonary emboli in a retrospective analysis.
6
Risk Factors
Venous thrombosis and pulmonary thromboembolism are a spectrum and therefore share many risk factors (
Table 65.1). Compared with deep venous thrombosis, PE is more strongly associated with major surgery, trauma, advanced age, myocardial infarction, and chronic heart failure, whereas malignancy and thrombophilia are stronger predictors of deep venous thrombosis.
7Over one-third of patients with deep venous thrombosis have a hypercoagulable state, most frequently factor V Leiden (activated protein C resistance, 47%), followed by prothrombin G20210A mutation (25%), multiple abnormalities (10%), protein C deficiency (7%), protein S deficiency (6%), and antithrombin III deficiency (5%).
8 In contrast, the risk of PE is only increased in patients with antithrombin deficiency or with prothrombin abnormality. The lack of risk for PE in patients with factor V Leiden despite the increased risk for deep venous thrombosis (so-called factor V Leiden paradox) has no clear explanation,
9 although it has been suggested that the deep venous thrombi with factor V Leiden are more distal and less likely to embolize.
10Less than 1% of surgical patients develop PE, with a higher rate after surgery for trauma and those who do not receive appropriate
prophylaxis.
11 The risk for pulmonary embolism typically peaks 2 to 3 weeks after trauma or surgery but may occur the first day. In 25% of patients, there are no known risk factors.
Among subjects with a malignant neoplasm, patients with pancreatic and gastric cancer, cancer of the large bowel, and women with ovarian cancers had the highest frequency of PE.
12,13,14 One percent to five percent of cancer patients have unsuspected PE.
15 Atrial fibrillation can be both a predisposing cause as well as an effect of chronic thromboembolic disease, and decreases overall survival.
16Systemic lupus erythematosus is associated with an increased risk of both PE and deep venous thrombosis, 13 to 20 times higher than the general population.
17 Exacerbation of chronic obstructive lung disease is associated with an increased risk of PE.
18
Clinical Findings
Clinically, PE is typically characterized by dyspnea and/or tachypnea. Cough and chest pain are also very common, though also nonspecific. Pleuritic chest pain is seen more commonly in those who have peripheral infarction with irritation of the visceral pleura. Lower extremity swelling can be an indicator of deep venous thrombosis. Fainting, syncope, and hemoptysis are also sometimes seen.
19Approximately 25% of patients dying suddenly have visited an emergency facility shortly prior to death, usually for dyspnea or chest pain, without a diagnosis.
20 In an additional one-third of patients dying suddenly, sudden death is the initial manifestation.
20
Pathology
From pathologists’ perspective, acute pulmonary thromboemboli are most frequently seen in the setting of autopsy. Chronic thromboemboli can be seen either in autopsy or occasionally for thromboendarterectomy. At autopsy, the evaluation of the pulmonary vascular tree should extend from the pulmonary trunk and main pulmonary arteries to lobar and segmental arteries. Features for the autopsy pathologist to consider are the point of origin, final localization, size and age of thromboemboli, the presence or absence of pulmonary infarction, and the underlying cardiac or other conditions. Although saddle emboli are invariably fatal, pulmonary emboli in cases of sudden death may be segmental, only involving lobar arteries.
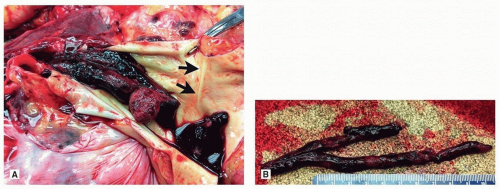
Grossly, a recent thromboembolus is usually cylindrical and takes the form of the originating vessel (usually a deep vein) and not the pulmonary artery (
Figs. 65.1 and
65.2). There is a wide variety of patterns of pulmonary embolism, but there is a predisposition to the right lung and lower lobes, and multiple emboli are the rule.
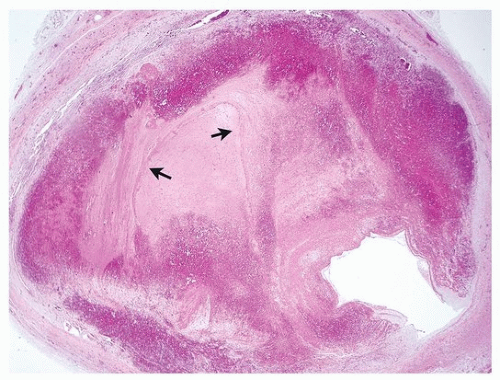
Thromboemboli encountered at autopsy are usually blunt with a smooth surface. Occasionally, impressions from the venous valves can be identified. Unlike postmortem clots, they protrude from the surface of the cut lung and are impacted in the distended artery. Lines of Zahn, a manifestation of blood flow, can be seen grossly and microscopically and confirm the antemortem nature. There is usually attachment to the vascular wall, but care should be taken while grossing the specimen not to dislodge the embolus while removing and dissecting the lung. If possible, sections of the thromboembolus with the attachment to the vessel should be submitted to histology. Obstruction of medium to large arteries may cause pulmonary infarction, which should also be noted and submitted to histology.
Microscopically, the intima contains collagen in all cases, with elastin in two-thirds, hemosiderin in one-half, and atherosclerosis in one-third.
27 Unusual histologic findings in patients with pulmonary thromboendarterectomy include pulmonary artery sarcoma metastatic tumor embolism and isolated pulmonary arteritis.
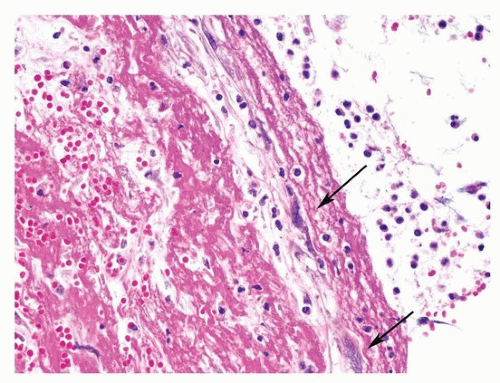
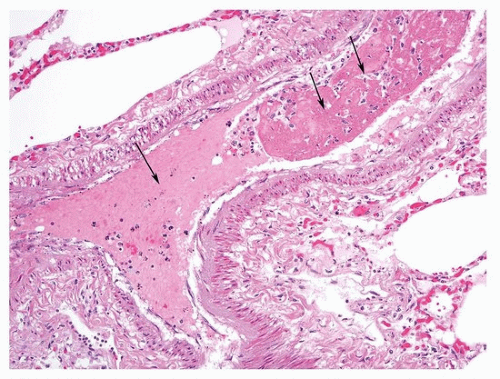
28The pathologist may be asked to estimate the age of the thromboembolic event in autopsy material, which may have clinical and legal implications. The task is especially important in the forensic field. Specific dating of the embolus is, however, impossible based on histologic grounds alone, and in selected cases, an estimate may only be attempted. Early histologic changes include endothelial activation of intima, ingrowth of myofibroblasts, formation of clefts lined by endothelial cells, centripetal ingrowth of capillaries and fibroblasts, deposition of reticulin, and endothelialization of the surface of the clot, while later events are characterized by hemosiderin and collagen deposition and recanalization.
Table 65.2 outlines general time lengths on the appearance of histologic findings. Emboli in different vessels can demonstrate various ages, indicating that the patient was sending emboli for some time prior to death.
Figures 65.2,
65.3,
65.4,
65.5,
65.6,
65.7 show progressive phases of organization, from acute thrombus to organizing and eventually recanalized thrombi.
Septic emboli differ from sterile thromboemboli by the presence of numerous neutrophils and typically the presence of intracellular bacteria, usually within neutrophils and macrophages (
Fig. 65.8). The major risk factors for septic pulmonary emboli are intravenous drug use, intravascular indwelling catheter, and purulent skin or soft tissue purulent infection, especially with
Staphylococcus. In addition to bacteria, septic emboli may also be due to
Candida, especially in intravenous drug addicts.
29


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access