Chapter 53 Pulmonary Embolism
Pulmonary embolism (PE) and deep venous thrombosis (DVT) comprise venous thromboembolism (VTE), a complex illness that warrants primary management or consultation by vascular medicine specialists. The Surgeon General estimates that PE causes between 100,000 and 180,000 deaths in the United States alone, and singles out PE as the most preventable cause of in-hospital death.1 Pulmonary embolism and DVT have attracted national attention among healthcare providers, policy makers, and the public. Advances in understanding PE’s epidemiology, prevention, diagnosis, and treatment are evolving at a rapid pace.
Epidemiology of Venous Thromboembolism
A cardinal misperception is that PE is much more benign than arterial cardiovascular diseases such as acute myocardial infarction (AMI). In fact, the case fatality rate for PE is much higher than that for MI, probably because PE is more difficult to detect and lacks wide application of definitive therapies such as thrombolysis or mechanical coronary revascularization. In an international PE registry at 52 institutions in 7 countries, the death rate was 17% after 3 months of follow-up. This registry had no exclusion criteria.2
The high mortality rate after PE is only the tip of the iceberg. Pulmonary embolism is associated with a multitude of adverse events. In a Dutch registry of 866 PE patients, a group of adverse events was tracked over time: death, recurrent VTE, arterial cardiovascular events, cancer, and chronic thromboembolic pulmonary hypertension (CTEPH). Some 30% of the Dutch PE cohort suffered adverse events within 1 year; the proportion increased to more than 40% after 2 years, and to more than 50% after 4 years.3 Chronic thromboembolic pulmonary hypertension4 used to be considered a rare complication of PE, but contemporary epidemiological studies indicate that it evolves in 1% to 3% of patients with acute PE. This complication causes marked dyspnea and makes patients vulnerable to sudden cardiac death. About half of patients with VTE will develop chronic venous insufficiency, also known as postthrombotic syndrome (see Chapter 55). This problem causes chronic leg swelling and discomfort, especially with standing. Brownish skin pigmentation can develop, especially in the medial malleolus. In extreme cases, venous ulceration may occur. Postthrombotic syndrome does not cause mortality, but does reduce quality of life for those who are stricken with it.5
Although the frequency of PE diagnosis among hospitalized patients is increasing in the United States, the mortality rate is decreasing. There were an estimated 127,000 cases of diagnosed PE in 1998, and this case load rose to 230,000 in 2005. These data reflect marked underestimates because most PEs are misdiagnosed as cardiac conditions such as AMI or sudden cardiac death. Nevertheless, the case fatality rate from PE decreased from 12.3% in 1998 to 8.2% in 2005. During this same time period, the estimated cost per hospitalized patient with PE almost doubled from $25,000 in 1995 to $44,000 in 2005.6
The incidence of VTE ranges between 1 and 2 per 1000 among adults in the United States. Prevalence is similar in men and women, and frequency of PE increases with age. There are about twice as many DVT as PE cases. About half are idiopathic (called primary PE) and half are provoked (called secondary PE) and occur after surgery, trauma, immobilization, or in association with cancer, birth control pill use, pregnancy, or postmenopausal hormone replacement. Certain genetic mutations such as factor V Leiden or the prothrombin gene mutation predispose to VTE (see Chapter 10).
In about half of cases, VTE is associated with acquired (Box 53-1) or inherited (Box 53-2) risk factors. Prior VTE increases the risk of recurrence. Risk factors for VTE are often modifiable and overlap with risk factors for coronary artery disease (CAD). Abstaining from cigarettes, maintaining lean weight, limiting red meat intake, and controlling hypertension might lower the risk of PE and DVT. Hospitalized patients at especially high risk include the elderly and those with cancer, congestive heart failure (CHF),7 or chronic obstructive pulmonary disease (COPD), as well as those undergoing surgery.8
![]() Box 53-1 Common Acquired Risk Factors for Venous Thromboembolism
Box 53-1 Common Acquired Risk Factors for Venous Thromboembolism
Prior venous thromboembolism (VTE)
Medical comorbidities, including obesity, heart failure, chronic kidney disease, chronic obstructive pulmonary disease (COPD), infection, and atherosclerosis
Oral contraceptives/hormonal replacement therapy
Indwelling central venous catheter
Lupus anticoagulant/antiphospholipid antibody syndrome
Three of four PEs occur in the outpatient setting. Outpatients presenting with PE who are at high risk for adverse outcomes include those with a history of congestive heart failure, cancer, and severe infection.9
In the prospective DVT FREE registry of 5451 patients,10 the most common acquired comorbidities were hypertension (50%), surgery within 3 months (38%), immobility within 30 days (34%), cancer (32%), and obesity (27%).
Cancer augments the risk of VTE11 through numerous mechanisms that include intrinsic tumor procoagulant activity and extrinsic factors such as chemotherapeutic agents and indwelling central venous catheters. Pancreatic, lung, gastric, genitourinary tract, and breast malignancies are associated with a particularly high risk of DVT and PE. In the California Cancer Registry,12 the highest incidence of VTE in cancer patients occurred during the first year of follow-up. The number of VTE events per 100 patient-years was 20 for pancreatic cancer, 11 for stomach cancer, 8 for bladder cancer, 6 for renal and uterine cancer, and 5 for lung cancer. Cancer chemotherapy increases levels of coagulation factors, suppresses anticoagulant and fibrinolytic activity, and directly damages the endothelium. Some patients with newly diagnosed VTE, especially idiopathic and unprovoked, harbor an occult cancer.13
Fatal PE associated with long-haul air travel has captivated the attention of the lay public. Although rare, the risk of massive PE increases progressively when the flight distance exceeds 5000 kilometers.14 There appears to be a dose-response relationship, with an estimated 18% higher risk of VTE for each 2-hour incremental increase in travel duration.15
Venous thromboembolism can adversely affect women’s health. Oral contraceptives,16 pregnancy,17 and postmenopausal hormone replacement therapy18 increase the risk of PE and DVT. Most oral contraceptives are second-generation agents that double or triple VTE risk. Newer third-generation agents have desogestrel or gestodene as the progestogen component and cause less acne and hirsutism, but they appear to cause acquired resistance to activated protein C (APC), with an incremental doubling or tripling of the VTE risk compared with second-generation contraceptives.
The antiphospholipid antibody syndrome is the most ominous acquired risk factor and is associated with arterial and venous thromboembolism as well as recurrent pregnancy loss. Autoantibodies bind to endothelial receptors to promote the release of tissue factor and suppress cell surface plasminogen activation.19
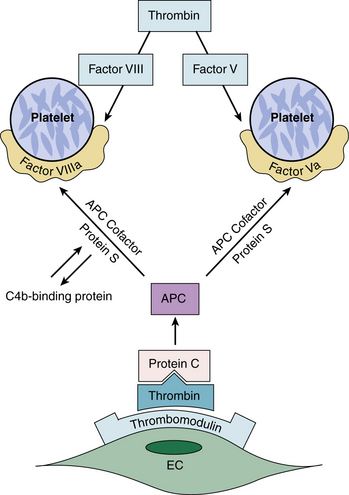
Thrombophilia is often inherited. A family history of VTE should be sought in all patients with DVT or PE. Whether laboratory testing should routinely be undertaken for patients with PE is controversial. The factor V Leiden mutation, a single-base mutation (substitution of A for G at position 506), is a common genetic polymorphism associated with APC resistance (Fig. 53-1). This genetic mutation is also a risk factor for recurrent pregnancy loss, probably due to placental vein thrombosis. The prothrombin gene mutation is a thrombophilic mutation identified in the 3′ untranslated region of the prothrombin gene (substitution of A for G at position 20210). This mutation causes increased prothrombin concentration and is associated with an increased risk of VTE. Use of oral contraceptives by patients with factor V Leiden or the prothrombin gene mutation is associated with a high risk of VTE.
Pathophysiology
Pulmonary embolism can manifest the following pathophysiological cardiopulmonary effects20: (1) increased pulmonary vascular resistance (PVR) due to vascular obstruction, neurohumoral agents, pulmonary artery baroreceptors, or increased pulmonary artery pressure; (2) impaired gas exchange due to increased alveolar dead space from vascular obstruction and hypoxemia from alveolar hypoventilation, low ventilation/perfusion (V/Q) units, and right-to-left shunting, as well as impaired carbon monoxide transfer owing to loss of gas exchange surface; (3) alveolar hyperventilation from reflex stimulation of irritant receptors; (4) increased airway resistance due to bronchoconstriction; and (5) decreased pulmonary compliance due to lung edema, lung hemorrhage, and loss of surfactant.
Hemodynamic alterations are common in patients with acute PE. Increased PVR and PAP cause RV shear stress and microinfarction. Increased myocardial shear stress can be quantified with brain natriuretic peptide (BNP) levels.21 Elevated troponin levels indicate myocardial ischemia and microinfarction.22 Myocardial ischemia and microinfarction are probably caused by two mechanisms: increased oxygen demand of the failing right ventricle and reduced coronary perfusion secondary to decreased systemic cardiac output.
Prevention
Pulmonary embolism is easier and less expensive to prevent than to diagnose or treat. A policy of routine VTE prophylaxis is cost-effective.23 Virtually all patients hospitalized for more than a day should receive prophylactic measures against VTE. Detailed guidelines for prevention of VTE are available from various consensus conferences. The most widely influential consensus is sponsored by the American College of Chest Physicians and recommends that “every hospital develop a formal strategy that addresses the prevention of VTE.”24 The type of prophylaxis strategy selected should match the level of risk for developing venous thrombosis (Table 53-1).
Table 53-1 Possible Prophylaxis Strategies
| Condition | Prophylaxis Strategy |
|---|---|
| General surgery | Enoxaparin 40 mg once daily |
| Dalteparin 2500 or 5000 units once daily | |
| UFH 5000 units bid/tid | |
| Total hip replacement | Enoxaparin 40 mg once daily |
| Fondaparinux 2.5 mg once daily | |
| Warfarin | |
| Total knee replacement | Enoxaparin 40 mg once daily |
| Fondaparinux 2.5 mg once daily | |
| Hip fracture surgery | Enoxaparin 40 mg once daily |
| Fondaparinux 2.5 mg once daily | |
| Neurosurgery | GCS and IPC PLUS UFH 5000 units bid or enoxaparin 40 mg once daily, PLUS predischarge venous ultrasound in patients with brain tumor |
| Trauma (not brain) | Enoxaparin 40 mg once daily |
| Thoracic surgery | GCS, IPC, and UFH 5000 units tid |
| Medical patients | UFH 5000 units SC tid |
| GCS or IPC | |
| Enoxaparin 40 mg once daily | |
| Dalteparin 5000 units once daily |
bid, twice daily; GCS, graduated compression stockings; IPC, intermittent pneumatic compression; SC, subcutaneous; tid, three times daily; UFH, unfractionated heparin.
Despite the availability of effective measures to prevent VTE, prophylaxis continues to be underused, even among high-risk hospitalized patients. Only half of high risk patients received prophylaxis in a survey of 15,000 acutely ill medical patients enrolled from 52 hospitals in 12 countries.25 In the even larger ENDORSE Study of 68,000 patients, with 32 countries participating from 6 continents, only 58% of surgical service and 40% of medical service patients received prophylaxis among those at moderate or high risk for VTE.26 However, failure to prevent in-hospital PE and DVT will no longer be tolerated by government regulators, hospital quality improvement committees, or the medicolegal system. For example, Medicare has stopped reimbursing hospitals for the incremental care needed to treat postoperative total hip or knee replacement patients who develop VTE.27 Whether this new policy is wise or equitable is debatable,28 but its influence in augmenting VTE prophylaxis and decreasing the rate of postoperative VTE is indisputable.
Prevention programs should be implemented to establish and enforce protocols that are streamlined and standardized.29 Computer-generated prompting can increase utilization of prophylactic measures.30,31 In a randomized trial of 2500 high-risk patients, a computer alert program increased physicians’ use of VTE prophylaxis and reduced the rate of symptomatic DVT and PE by more than 40%.32 At Brigham and Women’s Hospital (Boston, Mass.), the computer alert was upgraded from the initial single-screen version to a multiscreen set of alerts. The advanced algorithm increased use of VTE prophylaxis among physicians who had declined to order preventive measures following an initial traditional single-screen alert reminder.33 The multiscreen alert also provides a default option that automatically orders VTE prophylaxis unless the physician specifically “opts out.”
Many hospitals do not have the necessary electronic and information technology infrastructure to support sophisticated electronic alert systems for VTE prophylaxis. An alternative strategy uses a human alert. This system consists of a direct page from a hospital staff member to the attending physician when high-risk hospitalized patients are not receiving prophylaxis. In a multicenter randomized trial, this program of direct notification of the physician by a staff member tended to increase prophylaxis use and reduce the rate of symptomatic DVT and PE by about 20%, but the improvement was not statistically significant.34
Venous thromboembolism related events afflict 2 of every 100 acutely ill hospitalized medical patients. Most frequently affected are patients with heart failure, respiratory failure, pneumonia, and cancer. With probability modeling, symptomatic DVT, PE, and deaths from VTE will be halved if universal prophylaxis is used.35 Furthermore, long-term benefits will persist for at least 5 years by drastically reducing the number of cases of delayed complications such as postthrombotic syndrome and CTEPH.36
Mechanical prophylaxis measures use graduated compression stockings (GCS) and intermittent pneumatic compression (IPC). Graduated compression stockings increase venous blood flow and prevent perioperative venodilation of the legs. Intermittent pneumatic compression devices compress the veins more forcefully than GCS and also stimulate the endogenous fibrinolytic system. Mechanical VTE prophylaxis should be ordered for patients with active bleeding or extraordinarily high risk for major bleeding; however, pharmacological prophylaxis appears to be much more effective than IPC for preventing VTE in general surgery patients.37 Furthermore, in a large study of patients with major debilitating strokes, thigh-high GCS that were applied without pharmacological prophylaxis did not confer any protection against the development of proximal leg DVT.38
Fondaparinux, a pentasaccharide, is an anti–factor Xa agent and is effective in preventing VTE after orthopedic surgery in a fixed low dose of 2.5 mg daily. It also appears to markedly reduce VTE incidence among high-risk medical patients.39
In addition to anticoagulants, two novel pharmacological approaches appear promising for VTE prophylaxis: vitamin E supplementation and rosuvastatin. The Women’s Health Study randomized 39,876 women to receive 600 units of vitamin E or placebo. After a median follow-up of 10 years, there was a 21% reduction in VTE among women assigned to vitamin E. The reduction was most marked among women with VTE prior to randomization and in women with either the factor V Leiden or prothrombin gene mutation.40
The JUPITER Trial studied statin therapy for VTE prophylaxis among 17,802 apparently healthy men and women with both normal low-density lipoprotein (LDL) cholesterol levels and elevated high-sensitivity C-reactive protein (CRP) levels. They were randomized to receive rosuvastatin 20 mg per day or placebo. During a median follow-up period of 1.9 years, symptomatic VTE was reduced by 43% in the rosuvastatin group.41
In a study of middle-aged women undergoing surgery, the risk of VTE was substantially increased during the first 12 postoperative weeks, especially for those undergoing hip or knee replacement or cancer surgery.42 In the large RIETE Registry of VTE, the average time elapsed from surgery to VTE was 3 weeks.43 These findings suggest the need to extend the duration of VTE prophylaxis in high-risk patients beyond hospital discharge.
A large-scale randomized controlled trial tested the concept of extended-duration VTE prophylaxis in hospitalized acutely ill medical patients with reduced mobility.44 All patients initially received 6 to 14 days of enoxaparin 40 mg open-label VTE prophylaxis. Some patients completed this initial prophylaxis as outpatients. Those who remained at high risk were then randomized to enoxaparin 40 mg daily for 28 days or to placebo. The extended-duration enoxaparin prophylaxis group had a reduction in VTE from 4% to 2.5%, at a cost of an increase in major bleeding events from 0.3% to 0.8%. The benefits of extended duration enoxaparin appeared to be limited to women, patients older than 75 years, and those with marked immobility who did not have bathroom privileges. The trial was criticized because criteria for immobility were made stricter in a protocol amendment that was implemented about halfway through the trial.45
Diagnosis
Clinical Suspicion of Pulmonary Embolism
Assessment of the clinical pretest probability may improve the diagnostic accuracy in patients with suspected PE. Wells and coworkers46 have tested a bedside assessment score to estimate the clinical pretest probability for PE. The following clinical variables are required to calculate the score: signs or symptoms of DVT (3 points), no alternative diagnosis (3 points), a heart rate greater than 100 beats/min (1.5 points), immobilization or surgery within 4 weeks (1.5 points), a history of VTE (1.5 points), hemoptysis (1 point), and cancer (1 point). In this study, more than one third of the patients had a low Wells score of 2 or less. Pulmonary embolism was confirmed in only 2% of these patients. In contrast, half of the patients with a Wells score above 6 had PE diagnosed on further testing.
Pulmonary embolism should be suspected in hypotensive patients when (1) there is evidence of or predisposing factors for venous thrombosis, and (2) there is clinical evidence of acute cor pulmonale, such as distended neck veins, an S3 gallop, or an RV heave, especially if there is electrocardiographic (ECG) evidence of acute cor pulmonale manifested by a new S1Q3T3 pattern, new incomplete right bundle branch block, or T-wave inversion in V1 through V4 (Box 53-3).
Tests for Pulmonary Embolism
Arterial blood gas analysis
Neither room air arterial blood gases nor calculation of the alveolar-arterial oxygen gradient helps differentiate patients with a confirmed PE at angiography from those with a normal pulmonary angiogram.47 Therefore, arterial blood gases should not be obtained as a screening test in patients suspected of PE.
D-dimer
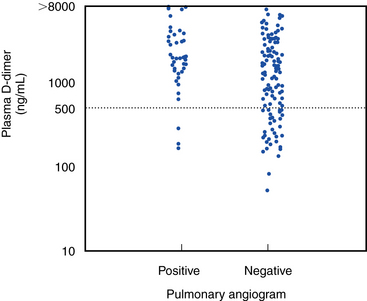
D-dimer is a specific proteolytic degradation product released into the circulation by endogenous fibrinolysis of a cross-linked fibrin clot. An abnormally elevated level of plasma D-dimer (>500 ng/mL) performed with a quantitative enzyme-linked immunosorbent assay (ELISA; Fig. 53-2) has a greater than 90% sensitivity for angiographically proven PE.48 Although elevated plasma concentrations of D-dimers are sensitive for the presence of PE, they are not specific. Levels are elevated for at least 1 week postoperatively and are increased in patients with pregnancy, MI, sepsis, cancer, or almost any other systemic illness. Therefore, this assay has greatest utility among outpatients or emergency department patients who have suspected PE but no coexisting acute systemic illness.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree