Essentials of Diagnosis
- Otherwise unexplained dyspnea, tachypnea, or chest pain.
- Clinical, electrocardiogram, or echocardiographic evidence of acute cor pulmonale.
- Positive chest computed tomography angiography scan with contrast.
- High-probability ventilation-perfusion lung scan or high-probability perfusion lung scan with a normal chest radiograph.
- Positive venous ultrasound of the legs with a convincing clinical history and suggestive lung scan.
- Diagnostic contrast pulmonary angiogram.
General Considerations
The term “venous thromboembolism” (VTE) encompasses both pulmonary embolism (PE) and deep venous thrombosis (DVT) and accounts for more than 250,000 hospitalizations per year in the United States. VTE constitutes one of the most common causes of cardiovascular and cardiopulmonary illnesses in Western civilization. PE causes or contributes to at least 50,000 deaths per year in the United States, a rate that has probably remained constant for the past three decades. However, a significant proportion of cases of PE remain undiagnosed, partly due to the variable nature of its clinical presentation and partly due to the lack of access of appropriate diagnostic testing modalities at the point of initial care. With the increasing use of computed tomographic pulmonary angiography (CT-PA) in mainstream clinical practice, a larger proportion of PE cases are now being diagnosed. In fact, the estimated incidence of PE has approximately doubled after the introduction of CT-PA in routine clinical practice, from 62.1 to 112.3 cases per 100,000 individuals. For those who survive PE, further disability includes the potential development of chronic pulmonary hypertension or chronic venous insufficiency. After a VTE event, patients and their physicians are concerned about the presence of an occult carcinoma, the risk of a recurrent PE after anticoagulation therapy has been discontinued, and whether the patients’ family members are at risk for VTE.
Etiology
“Primary” PE occurs in the absence of surgery or trauma. Patients with this condition often have an underlying hypercoagulable state, although a specific thrombophilic condition may not be identified. A common scenario is a clinically silent tendency toward thrombosis, which is precipitated by a stressor such as prolonged immobilization, oral contraceptives, pregnancy, or hormone replacement therapy. Recently, there has been an increased appreciation of the risks of VTE among patients with medical illnesses, including cancer (which itself may be associated with a hypercoagulable state), congestive heart failure, and chronic obstructive pulmonary disease.
The prevalence of “secondary” PE is high among patients undergoing certain types of surgery, especially orthopedic surgery of the hip and knee, gynecologic cancer surgery, major trauma, and craniotomy for brain tumor. PE in these patients may occur as late as a month after discharge from the hospital.
A thorough history should be obtained, including history of prior VTE, family history of VTE, history of frequent miscarriages, past history of cancer, recent history of heparin use (suggestive of heparin-induced thrombocytopenia), and history of prothrombotic conditions (myeloproliferative disease, nephrotic syndrome, collagen-vascular disease, and congestive heart failure). An acquired or inheritable risk factor is found in 50% of patients who present with an initial VTE. Principal thrombophilic risk factors for VTE are listed in Table 29–1. The two most common genetic mutations that predispose to VTE are the factor V Leiden and the prothrombin gene. Both are autosomal dominant. Whether factor V Leiden predisposes to recurrent VTE after anticoagulation is discontinued remains controversial. The prothrombin gene mutation is associated with an increased risk of recurrent VTE after discontinuation of anticoagulation, especially in patients who have coinherited the factor V Leiden mutation.
Common Factor V Leiden Prothrombin gene mutation Anticardiolipin antibodies (including lupus anticoagulant) as a feature of the antiphospholipid antibody syndrome Hyperhomocysteinemia (usually due to folate deficiency) Uncommon Antithrombin III deficiency Protein C deficiency Protein S deficiency Mutations of cystathionine β-synthase or methylene tetrahydrofolate reductase (MTHFR) High concentrations of factors VIII or XI (or both) |
Laboratory screening for inherited thrombophilia does not always impact the treatment plan and therefore is not always indicated. Regardless of whether a risk factor is identified, just the history of having had a VTE event is the most significant risk factor for predicting future recurrence. Screening for deficiencies of antithrombin III, protein C, and protein S is a low-yield strategy that produces positive findings in less than 5% of patients. Heparin decreases the antithrombin III level, whereas warfarin, pregnancy, and oral contraceptives decrease the protein C and S levels, thereby resulting in potentially spurious diagnoses of these hypercoagulable states. There are no data that support screening for or treating hyperhomocysteinemia in the setting of VTE. A positive finding of inherited thrombophilia does not necessarily imply that the patient is at higher risk for recurrent VTE or would benefit from extended duration of anticoagulation. Therefore, the 2012 American College of Chest Physicians (ACCP) guidelines do not find that testing for inherited thrombophilic disorders should be a major determinant of VTE treatment. However, in patients with a strong family history of VTE, or based on strong patient preferences, screening can be considered for the patient and first-degree relatives, particularly if it may impact decisions about oral contraceptive use.
In contrast to screening for inheritable disorders, because malignancy is a hypercoagulable state and because VTE may be the first presenting symptom of malignancy, age-appropriate cancer screening after VTE is prudent.
PE poses a special threat for women because VTE is associated with the use of oral contraceptives, pregnancy, and hormone replacement therapy.
One-third of pregnancy-related VTE occurs postpartum. The risk of DVT is present throughout pregnancy and is highest during the third trimester. After delivery, two of the most important risk factors for VTE are increased maternal age and cesarean section. Emergency cesarean section increases the VTE risk by about 50% compared with elective cesarean section.
Among women with a history of VTE during pregnancy or puerperium in one study, the prevalence of factor V Leiden was 44% and the prevalence of the prothrombin gene mutation was 17%. Compared with controls, the Leiden mutation increased the risk of VTE ninefold, and the prothrombin gene mutation increased the risk by a factor of 15. The combination of the Leiden and prothrombin gene mutations increased the VTE risk to more than 100 times that seen in the controls. Irrespective of factor V Leiden, pregnancy itself causes hypercoagulability because it induces a relative state of activated protein C resistance.
Hormone replacement therapy also predisposes to VTE. In 1996, three separate large data sets implicated hormone replacement therapy as doubling, tripling, or even quadrupling the risk of VTE. As with oral contraceptives, the risk of VTE peaks during the first year of hormone replacement therapy.
Clinical Findings
PE is often difficult to diagnose due to the variable nature of its clinical presentation. Acute PE can manifest clinically as massive, submassive, or nonmassive based on the severity of the clinical presentation, although this distinction is somewhat vague and does not necessarily correlate with clinical outcomes. Acute massive PE is a life-threatening condition characterized by sudden onset of chest pain, hypotension, hypoxemia, and distended neck veins, and it needs to be differentiated quickly from other potentially lethal conditions including acute myocardial infarction, tension pneumothorax, or pericardial tamponade. Despite the availability of radionuclide lung scanning, chest computed tomography (CT) scanning, and pulmonary angiography, many pulmonary emboli are not discovered until postmortem examination. Appreciation of the clinical settings that make patients susceptible to PE and maintenance of a high degree of clinical suspicion are, therefore, of paramount importance.
The most common symptoms or signs of PE are nonspecific: dyspnea, tachypnea, chest pain, or tachycardia. Patients with life-threatening or massive PE are apt to have dyspnea, syncope, or cyanosis rather than chest pain. Less than one-third of patients with PE have symptoms of a DVT. Massive PE should be suspected in hypotensive patients who have evidence of, or predisposing factors for, venous thrombosis and clinical findings of acute cor pulmonale (acute right ventricular failure), such as distended neck veins, an S3 gallop, a right ventricular heave, tachycardia, or tachypnea. The definition of massive PE proposed by the American Heart Association is: an acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support, not due to a cause other than PE, such as arrhythmia, hypovolemia, sepsis, or left ventricular dysfunction), pulselessness, or persistent profound bradycardia (heart rate < 40 bpm with signs or symptoms of shock). Submassive PE has been defined as an acute PE without systemic hypotension (systolic blood pressure > 90 mm Hg) but with either evidence of right ventricular dysfunction or myocardial necrosis. Low-risk or nonmassive PE is defined as an acute PE without the presence of hemodynamic instability, right ventricular dysfunction, or myocardial necrosis.
Patients with severe chest pain or hemoptysis usually have anatomically small PE near the periphery of the lung. This is where innervation is greatest and where pulmonary infarction is most likely to occur from a dearth of collateral bronchial circulation.
Decision algorithms have been developed to help determine the probability of PE based on clinical symptoms and signs and to guide the selection of diagnostic tests in order to make a rapid and accurate diagnosis and thus initiate treatment without delay. To this end, an integrated diagnostic approach for cases of suspected PE, as shown in Figure 29–1, has been shown to be useful. The diagnostic workup should begin by quantifying the clinical suspicion with a precise clinical scoring algorithm.
Traditionally, the clinical likelihood of PE has been estimated subjectively by “gestalt” as low, moderate, or high. However, to better define the clinical probability of PE in a given patient, quantitative clinical scoring systems have been devised in order to provide the clinician with a standardized and objective approach.
One of the most widely used clinical scoring systems is the Ottawa Scoring System (also known as the modified Wells index), which has a maximum of 12.5 points (Table 29–2). The greatest emphasis is placed on the presence of signs or symptoms of DVT (3 points) and whether an alternative diagnosis is unlikely (3 points). If the score exceeds 4 points, the overall likelihood of PE being confirmed with imaging tests is 41%. However, if the score is 4 points or less, the likelihood of PE is only 8%. Although this scoring system has the advantage of simplicity and rapidity, it offers a somewhat subjective approach with regard to the important, heavily weighted category of “alternative diagnosis unlikely.”
Signs or Symptoms | Points |
|---|---|
Hemoptysis | 1.0 |
Malignancy (on treatment, treated in the last 6 months or palliative) | 1.0 |
Heart rate > 100 bpm | 1.5 |
Immobilization or surgery in the previous 4 weeks | 1.5 |
Previous DVT or PE | 1.5 |
Clinical signs and symptoms of DVT (minimum of leg swelling and pain with palpation of the deep veins) | 3.0 |
An alternative diagnosis is less likely than PE | 3.0 |
Risk level | Score |
Low | 0–1 |
Intermediate | 2–6 |
High | ≥7 |
In clinical settings such as outpatient clinics or emergency departments where the overall prevalence of PE is low but many patients have symptoms such as chest pain or shortness of breath, the Pulmonary Embolism Rule-out Criteria (PERC) may be used to exclude a PE without additional diagnostic testing. The eight factors that constitute the PERC are age < 50 years, heart rate < 100 bpm, oxygen saturation ≥ 95%, no hemoptysis, no estrogen use, no prior history of PE or DVT, no unilateral leg swelling, and no surgery or trauma needing hospitalization within the past 4 weeks. If a patient does not have any of the PERC criteria and has a low clinical suspicion of PE using clinical gestalt or the modified Wells index, then the diagnosis of PE can likely be excluded without additional diagnostic testing. This approach has been validated in a multicenter cohort study of > 8000 emergency department patients, but it should only be used in a setting where the prevalence of acute PE is low.
Electrocardiograms (ECG) may show evidence of acute cor pulmonale, manifested by a new S1 Q3 T3 pattern, new incomplete right bundle branch block, right axis deviation, or right ventricular ischemia or strain with ST segment depressions in the right precordial leads. However, these changes are usually only seen in patients with acute massive PE, and therefore, absence of these ECG changes in a patient with suspected PE should not preclude additional diagnostic testing. Some ECG findings that have been associated with an adverse prognosis in patients with acute PE include atrial arrhythmias, right bundle branch block, Q waves in the inferior leads III and aVF, and ST segment changes and T-wave inversions in the precordial leads.
Neither measurement of room air arterial blood gases nor calculation of the alveolar-arterial oxygen gradient is useful in excluding the diagnosis of PE. Among patients in whom PE is suspected, neither test helped differentiate patients with a confirmed PE at angiography from those with a normal pulmonary angiogram. Therefore, arterial blood gases should not be obtained as a screening test for suspected PE.
Endogenous fibrinolysis, although ineffective in preventing PE, almost always causes the release of d-dimers from fibrin clot in the presence of established PE. However, an elevated d-dimer test is not specific and may occur in a variety of other conditions including acute comorbid illnesses, such as acute myocardial infarction, metastatic cancer, sepsis, or recent surgery. A normal (< 500 ng/mL) result virtually excludes the diagnosis of PE: among patients with a normal d-dimer level, the likelihood of PE is < 5%. Despite its sensitivity, in patients with a very high clinical suspicion of PE, the d-dimer should not be used in isolation to exclude PE. The combination of low clinical suspicion, ideally quantified with a validated scoring system, and a normal d-dimer enzyme-linked immunosorbent assay makes PE exceedingly unlikely.
Screening for troponins is now the standard blood test for cardiac injury, and it is obtained routinely when acute myocardial infarction or unstable angina is suspected. Circulating troponin indicates irreversible myocardial cell damage and is much more sensitive than creatine kinase or its myocardial muscle isoenzyme. Cardiac markers of injury should not be used, however, as a primary diagnostic test for acute PE. Nevertheless, elevation of cardiac troponin is an adverse prognostic factor in patients with acute PE and is associated with a markedly increased mortality rate and requirement for inotropic support and mechanical ventilation. Troponin elevation also correlates with ECG evidence of right ventricular strain. This suggests that release of troponin from the myocardium during PE may result from acute right ventricular microinfarction due to pressure overload, impaired coronary artery blood flow, or hypoxemia caused by the PE. A meta-analysis of 20 observational studies showed that patients with acute PE with elevated troponin T or troponin I levels had a fivefold higher risk of short-term mortality and a ninefold increased risk of death due to PE.
As with cardiac troponins, measurement of BNP levels or N-terminal proBNP (NT-proBNP) levels should not be used to diagnose an acute PE. However, elevation of BNP or NT-proBNP levels may be a reflection of right ventricular dysfunction in the setting of an acute PE and therefore may have prognostic value. In a meta-analysis of 16 studies of patients with acute PE, in-hospital or short-term mortality was increased sixfold in those with a BNP level > 100 pg/mL and 16-fold among those with an NT-proBNP level > 600 ng/L.
Chest radiography can help exclude diseases such as lobar pneumonia, pneumothorax, or cardiogenic pulmonary edema, which can have clinical presentations that mimic acute PE. However, patients with these disorders can also have concomitant PE.
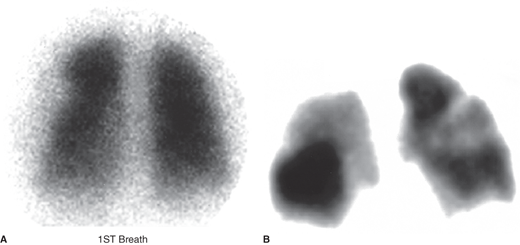
This study has served as the principal diagnostic imaging test for PE but is now with increasing frequency being superseded by chest CT scanning. Lung scanning is most useful when unequivocally normal or when highly suggestive of PE (Figure 29–2). Neither intermediate nor low-probability scans (in the presence of high clinical suspicion) exclude PE. For example, with the combination of a low-probability scan and high clinical suspicion for PE, the likelihood of PE is 40%. When lung scanning is performed, the ventilation scan is being used less frequently than previously because its contribution to the diagnostic decision is only marginally better than the combination of a perfusion scan and chest radiograph.
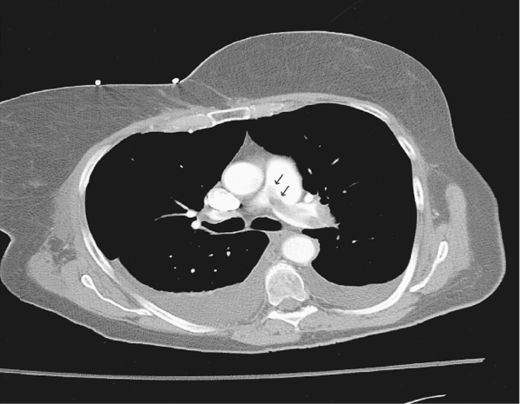
The chest CT is diagnostic of PE when an intraluminal pulmonary arterial filling defect is surrounded by contrast material. CT scanning has two major advantages over lung scanning: (1) directly visualizing thrombus and (2) establishing alternative diagnoses on CT images of the lung parenchyma that are not evident on a chest film.
Conventional chest CT scanning relies on imaging a series of consecutive sections of the chest. With the introduction of spiral chest CT scanning, patients can be scanned continually. As patients are advanced through the spiral CT scanner, the x-ray source and single-row detector array rotate around them. These scans are performed during a single breath-hold, thereby eliminating respiratory motion artifact that previously limited thoracic imaging. Overlap data from adjacent slices are acquired, thus reducing the possibility of missed pathology. Scans are performed in less than 30 seconds, and excellent vascular opacification with the contrast agent can usually be achieved (Figure 29–3). However, the major limitation has been failure to detect PEs beyond the third-order pulmonary arterial branches.
Further innovations occurred with the introduction of multidetector CT scanners, which acquire four slices simultaneously during each rotation of the x-ray source. Multidetector CT improves resolution from 5 mm to 1.25 mm and allows for better visualization of subsegmental vessels. Compared with conventional spiral CT, the sensitivity of multirow detector scanners for the diagnosis of acute PE increased from about 70% to 80–90%. In the PIOPED II study, which used multidetector CT angiography, the sensitivity and specificity were 83% and 96%, respectively. The positive predictive value was 96% with a concordant clinical assessment. The addition of CT venography to image DVTs improves the sensitivity from 83% to 90% with no change in specificity. In order to answer concerns that CT angiography could miss clinically important PEs in the branch pulmonary arteries, another randomized trial directly compared CT angiography with V̇/Q̇ scanning. In this study, CT was found to be noninferior. PE was diagnosed in more patients in the CT group than in the V̇/Q̇ scanning group. The risk of VTE during 3-month follow-up after having a negative imaging study was 0.4% for CT and 1% for V̇/Q̇ scan.
Occasionally, determining whether a pulmonary embolus represents the residua of prior PE or a new event may be challenging. A systematic review of the literature has shown that over half of patients with PE will still have a defect on either CT scan or radionuclide imaging 6 months after diagnosis.
In combination with color Doppler imaging, venous ultrasonography is known as duplex sonography and is the principal diagnostic imaging test for suspected acute DVT. Sonographic evaluation uses compression ultrasound along the full length of the femoral, popliteal, and calf veins. The transducer is held transverse to the vein and, normally, the vein collapses with gentle manual compression. The compressed vein appears as if it is winking. The main criterion for diagnosing DVT is lack of compression of a deep vein. This diagnosis can be confirmed by direct visualization of thrombus on ultrasound or by abnormal venous flow on Doppler examination (eg, loss of physiologic respiratory variation or loss of the expected augmentation of blood flow during calf compression).
Among symptomatic patients, duplex sonography is very accurate, with high sensitivity and specificity. Its sensitivity decreases when assessing asymptomatic patients. Major limitations include an inability to image pelvic vein thrombosis directly, lower sensitivity for diagnosing isolated calf DVT, and difficulty diagnosing an acute DVT superimposed upon a chronic one. Magnetic resonance imaging may be useful under these circumstances.
Importantly, many patients with PE do not have evidence of leg DVT, probably because the thrombus has already embolized to the pulmonary arteries. Therefore, PE is not necessarily ruled out if the clinical suspicion is high and imaging evidence of DVT is lacking.
Echocardiography should not be used routinely to diagnose suspected PE because most patients with PE have normal echocardiograms. However, the echocardiogram, like the troponin level, is an excellent tool for risk stratification and prognostication. Echocardiography is useful diagnostically when the differential diagnosis includes pericardial tamponade, right ventricular infarction, and dissection of the aorta as well as PE.
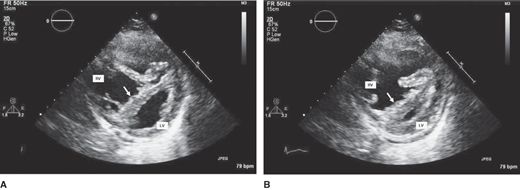
Imaging a normal left ventricle in the presence of a dilated, hypokinetic right ventricle strongly suggests the diagnosis of PE (Figure 29–4). The presence of right ventricular mid-free wall akinesis with sparing of the apex (the McConnell sign) has been found to be highly specific for acute PE. Echocardiographic findings in PE patients are summarized in Table 29–3.
Figure 29–4.
Parasternal short-axis views of the right ventricle (RV) and left ventricle (LV) in (A) diastole and (B) systole. Diastolic and systolic bowing of the interventricular septum (arrows) into the LV is evident, which is compatible with RV volume and pressure overloads, respectively. The RV is appreciably dilatated and markedly hypokinetic, with little change in apparent RV area from diastole to systole. PE, small pericardial effusion.
Abnormal Finding | Description |
|---|---|
Right ventricular dilatation and hypokinesis | Associated with leftward septal shift; the ratio of the RVEDA to LVEDA exceeds the upper limit of normal (0.6). Associated with right atrial enlargement and tricuspid regurgitation. |
Septal flattening and paradoxical septal motion | Right ventricular contraction continues even after the left ventricle starts relaxing at end-systole; therefore, the interventricular septum bulges toward the left ventricle. |
Diastolic left ventricular impairment with a small difference between left ventricular area during diastole and systole, indicative of low cardiac output | Due to septal displacement and reduced left ventricular distensibility during diastole; consequently, Doppler mitral flow exhibits a prominent A wave, much higher than the E wave, with an increased contribution of atrial contraction to left ventricular filling. |
Direct visualization of PE | Only if PE is large and centrally located; much more easily visualized on transesophageal than on transthoracic echocardiography. |