Chapter 77 Pulmonary Complications of Hematopoietic Stem Cell Transplantation
Posttransplant Pulmonary Complications
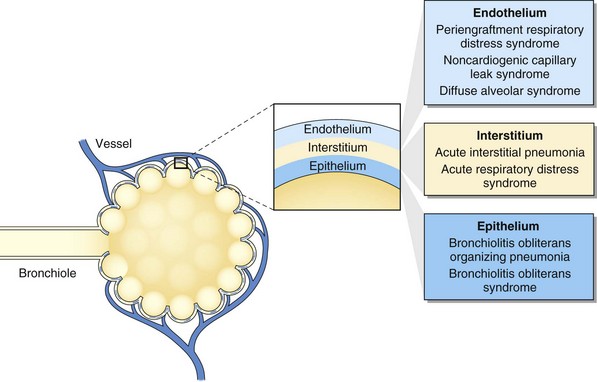
Pulmonary complications after HSCT are common, with an incidence of 40% to 60% and with up to one third of recipients requiring intensive care after transplantation. Respiratory failure is the most common cause of critical illness, and pneumonia is the leading infectious cause of death after HSCT (Figure 77-1). Pulmonary complications can occur early or late in the posttransplant course, can have infectious and noninfectious etiologies, and can present with assorted radiographic findings. The pulmonary complications of HSCT also vary depending on the indication for, type of, and preparative regimen preceding HSCT.
Risk Factors for Pulmonary Disease
Relapse status at transplant and donor-recipient HLA mismatching or nonidentity are risk factors for pulmonary complications and mortality after HSCT (Box 77-1). Active phase of malignancy, age over 21 years, and receipt of HLA-nonidentical donor marrow are risk factors for respiratory failure after HSCT.
Box 77-1
Risk Factors for Pulmonary Complications After Hematopoietic Stem Cell Transplantation (HSCT)
Infectious Complications
Supportive care in the posttransplant period has changed the microbiology of pneumonia. Prophylactic administration of trimethoprim-sulfamethoxazole (TMP-SMX), antivirals, antifungals, and fluoroquinolones has decreased the incidence of Pneumocystis jirovecii, CMV, herpes simplex, and Candida albicans infections (Box 77-2). Resistant gram-negative and gram-positive bacteria, viruses, and other fungi remain important pathogens.