PULMONARY ATRESIA WITH INTACT VENTRICULAR SEPTUM
Introduction
Pulmonary atresia with intact ventricular septum (PA-IVS) is a rare congenital heart defect (CHD) that accounts for <1% of all CHDs.1 PA-IVS is different from pulmonary atresia with ventricular septal defect (VSD). The latter is part of tetralogy of Fallot (TOF) spectrum of disorders and is discussed elsewhere in this book. PA-IVS comes with unique set of anatomic and hemodynamic challenges that are described in this chapter. Management of babies with this condition has evolved in the past 2 decades as reviewed elsewhere from time to time,2–6 with transcatheter therapy during the neonatal period emerging as a viable treatment option over surgery in selected patients.
Pathologic Anatomy
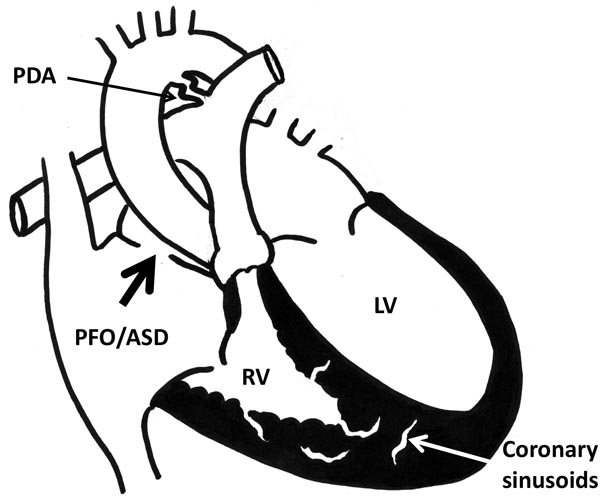
PA-IVS is defined by atresia of the pulmonary valve (PV) and an intact ventricular septum. Components of this defect include small tricuspid valve (TV), small but hypertrophic right ventricle (RV), and atresia of the PV (Figure 34.1). It is usually a membranous atresia of the PV rather than muscular atresia.7 Right ventricular outflow tract ([RVOT]; the infundibular portion) is usually patent in this condition. Main pulmonary artery (MPA) and branch pulmonary arteries (PAs) are usually well developed and adequate in size with a patent ductus arteriosus (PDA) at birth. In view of pulmonary atresia, presence of a nonrestrictive atrial level communication, such as patent foramen ovale (PFO) or atrial septal defect (ASD), is essential for right atrium (RA) to decompress. Similarly, PDA is essential to provide pulmonary blood flow (PBF) immediately after birth. Each of the essential components of this lesion is discussed below.
Figure 34.1. Schematic diagram shows PA-IVS. Large arrow in RA points to PFO/ASD, which is essential to allow egress of right atrial blood and avoid systemic venous congestion or obstruction. Note, coronary sinusoids are shown in the RV myocardium. PDA is shown. LV, left ventricle.
Tricuspid Valve (TV)
TV is usually smaller than normal. Annulus diameter has become an indicator or predictor of several factors: (1) adequacy of RV; (2) future potential for RV growth and (3) presence of RV-dependent coronary circulation (RV-DCC). All of these are described in the passages that follow. In addition, TV itself may be abnormal in anatomy with tethering and stenosis. It is of note that apparent diminished mobility of TV leaflets in neonatal echocardiogram may be secondary to restriction from not having enough room to open in a small RV and the valve leaflets will exhibit better mobility as the RV grows in size after neonatal treatment and further growth. TV may be dysplastic and may have Ebstein-like anomaly. Tricuspid regurgitation of varying severity is usually present. Doppler estimate of tricuspid regurgitation pressure gradient will help to assess RV systolic pressure.
RV
Varying degrees of RV hypoplasia is an important characteristic of PA-IVS. Conceptually, RV cavity consists of 3 portions, namely inlet, trabecular and outlet (or infundibular) portions. Based on the number of portions present, RV morphology can be categorized into 3 major types8: tripartite, bipartite, and unipartite. In tripartite RV, consisting of inlet, trabecular and outlet portions of the RV, the ventricle is nearly normal in size. This type of RV will be expected to enlarge provided the TV annulus size is adequate. In bipartite RV, the inlet and outlet portions are present and the trabecular portion is obliterated or absent. There is moderate hypoplasia of the RV in this type. Adequacy of RV with growth is variable. In unipartite RV, only the inlet portion is present. The RV is severely hypoplastic with high pressure.
Coronary Circulation
Coronary sinusoids originating from RV and connecting to native coronary arteries (CAs) are important associations present in a small number of patients. These coronary sinusoids originate from the hypertensive RV cavity and connect with native CAs arising normally from the aorta. In some patients with coronary sinusoids, aortic end of the CAs may be either atretic or stenotic; this leaves the coronary circulation dependent upon its connections with the RV cavity via coronary sinusoids. Such coronary circulation is called RV-DCC. Neonatal diagnostic workup should include confirming the presence or absence of RV-DCC. Presence of RV-DCC is a contraindication to perform 2-ventricle repair because decompressing the RVOT will reduce RV pressure and, thus, reduce coronary blood flow that is dependent on high pressure in the RV. In some centers, this is taken as an indication for heart transplantation.
The morphological heterogeneity due to severity of each of the components with their respective hemodynamic implications provides a great challenge in evaluation and clinical decision-making for the cardiologists, interventional cardiologists and surgeons.9,10
Embryology
During embryogenesis, PA-IVS is postulated to occur after development of ventricular septum, which is later than the development of pulmonary atresia with VSD.7 During early embryonic period of normal heart development, coronary sinusoids are usually present in communication with ventricular cavity. These involute by the later part of embryonic period. In PA-IVS, there is very high pressure in RV due to pulmonary atresia which hinders the spontaneous closure of embryonic coronary sinusoids and, therefore, they tend to persist.11
Pathophysiology
PDA is the sole source of PBF in pulmonary atresia. Systemic venous return to RA needs an adequate egress. Due to an intact ventricular septum, the only egress for systemic venous return is at the atrial level. Adequate size PFO or ASD is essential for RA to decompress via an obligatory right-to-left shunt at atrial level. The deoxygenated blood from RA shunts via PFO/ASD and mixes with fully saturated pulmonary venous blood in left atrium (LA) causing “central” mixing and central cyanosis.
RV systolic pressure in PA-IVS is extremely high, usually 200% of the left ventricular pressure. The amount of blood that enters RV has to return back via TV. Therefore, there is significant tricuspid regurgitation. Coronary sinusoids when present have to be distinguished into 2 categories, that is, RV-DCC or not. If RV-DCC exists, RV pressure should not be reduced by opening the atretic PV for the fear of causing myocardial ischemia. Such patients either undergo repair via single-ventricle pathway or receive heart transplantation.
Natural History
Without intervention, PA-IVS is a fatal condition, even though rare cases of survival to young adulthood have been reported.12,13 Without treatment, 50% die within 2 weeks of age and about 85% die by 6 months of age. Causes of death are extreme cyanosis and metabolic acidosis.14
Clinical Features
Typical presenting symptom is cyanosis in the first few days of life as the PDA begins to close. Severity of cyanosis is proportional to the amount of PBF, which in turn is secondary to size and flow via PDA. Early on, babies appear apparently normal except for cyanosis.15 Obligatory right-to-left shunt at the atrial level adds to the severity of cyanosis. If the PFO is restrictive, features of systemic venous congestion may occur because RA is unable to decompress. Respiratory distress and metabolic acidosis ensue with continued severe hypoxia.
Physical examination findings will include a cyanotic baby with absence of RV impulse and presence of palpable left ventricular impulse. Respiratory distress may be present. Liver enlargement with or without peripheral edema or ascites may be present due to systemic venous congestion should the PFO is restrictive. Auscultation will reveal single S2 and a murmur of tricuspid regurgitation. PDA murmur is usually not heard. Differential diagnosis for this presentation will include several lesions such as tricuspid atresia, Ebstein’s anomaly, and critical pulmonary stenosis (PS).16,17
Noninvasive Evaluation
Chest X-Ray
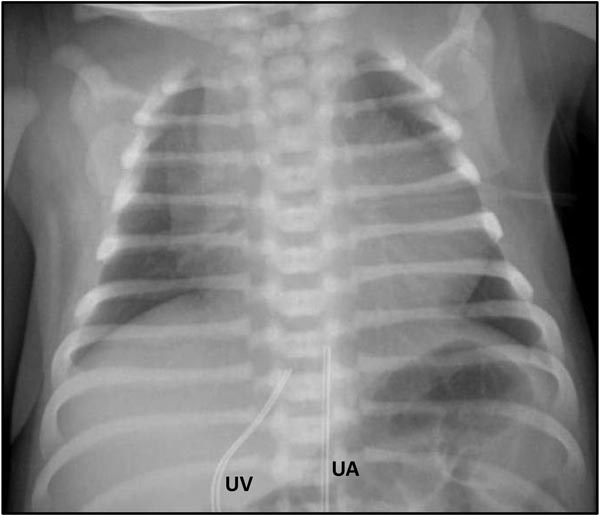
The chest x-ray (Figure 34.2) may show a normal-sized heart or possible cardiomegaly if there is significant right atrial enlargement. The MPA segment may be concave with diminished pulmonary vascular markings. However, if PBF is high through a large PDA, pulmonary vascular markings may be normal or even increased in some cases. This may be accompanied by relatively higher systemic oxygen saturation above mid-80s.
Figure 34.2. Chest x-ray of a 1-day-old baby with PA-IVS receiving PGE1 infusion shows moderate cardiomegaly, right atrial enlargement and decreased pulmonary vascular markings. Umbilical arterial (UA) and venous (UV) lines are in place.
ECG
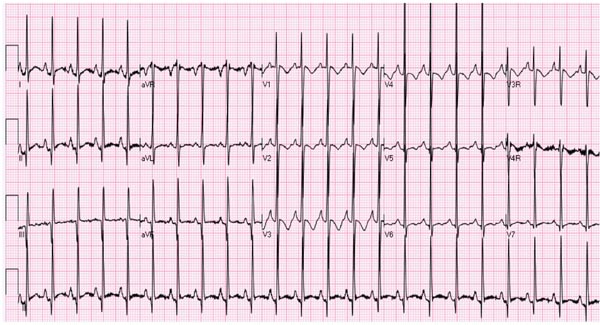
ECG shows normal QRS axis, right atrial enlargement, left ventricular hypertrophy with monopartite type (Figure 34.3) and possible RV hypertrophy with tripartite type. Similar ECG findings may be present in cyanotic newborns with tricuspid atresia as well, but a “superior” QRS axis in tricuspid atresia may distinguish PA-IVS from tricuspid atresia. ST segment depression with or without T-wave inversion may be seen secondary to subendocardial ischemia secondary to high pressure in RV cavity. Ischemia from coronary sinusoids is not usually evident in a newborn.
Figure 34.3. ECG of the same baby in Figure 34.2 shows +40° frontal plane axis, right atrial enlargement and left ventricular dominance.
Echocardiogram
Two-dimensional (2D) echocardiogram is diagnostic. Apart from recognition of the overall anatomy and the diagnosis, several measurements should be obtained, including size of TV, RV, PV annulus, PAs, size of PFO/ASD and PDA. Color Doppler imaging of RV wall will provide clues about the presence of coronary sinusoids. Specific attention should be paid to the following aspects of the echocardiogram:
- Adequacy of PFO: Since the PFO is the only egress for blood from RA, the adequacy of PFO should be assessed. Small opening on subcostal 2D imaging with turbulent flow and high Doppler velocity are suggestive of PFO obstruction. RA may be enlarged more than the LA when there is some restriction to the obligatory right-to-left shunt. However, it is rare to find the PFO to be inadequate in a neonate with this condition.
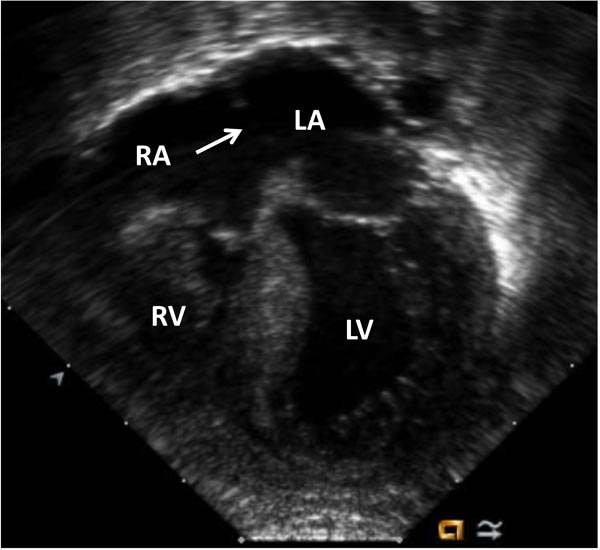
- TV: Size of TV annulus is an important measurement (Figure 34.4). TV annulus size is directly proportional to the development of RV size, coronary sinusoids and presence of RV-DCC. It is customary to report TV annulus diameter as z-score, which represents the number of standard deviations above or below the mean diameter expected for the baby’s body surface area. TV annulus diameter worse than (−3) z-scores is associated with presence of coronary sinusoids and is more likely to require repair as a single-ventricle pathway. More importantly, a TV z-score greater than (−3) has been associated with successful biventricular repair.18 Severity of tricuspid stenosis and regurgitation should also be assessed.
Figure 34.4. Echocardiogram (apical 4-chamber view) of the same baby in Figure 34.2. Severe TV hypoplasia with severely hypoplastic RV with thick myocardium is seen. Arrow indicates to the location of nonrestrictive atrial defect. Subsequent imaging with color Doppler showed random color signals in RV myocardium raising suspicion of coronary sinusoids – confirmed by angiogram in Figure 34.5. LA, left atrium; LV, left ventricle; RA, right atrium.
- Mitral valve (MV) annulus: The ratio of TV to MV annulus diameters is another helpful parameter in determining single versus biventricular repair. A TV/MV annulus ratio >0.5 has been shown to be a predictor of successful biventricular repair in PA-IVS patients.19
- RV: This cavity is considered to be tripartite when it consists of 3 portions—inlet, body and outlet. In PA-IVS, RV may be bipartite or unipartite. Bipartite RV may have either inlet and outlet portions only or trabecular and outlet portions only. These are associated with moderately severe RV hypoplasia where unipartite RV, usually containing only the inlet portion, is associated with severe RV hypoplasia and is likely to require repair via univentricular route.8 The echocardiographer should look for presence of coronary sinusoids in the myocardium using color Doppler mapping and any possible communications to RV cavity.9,11
- PV

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree