(1)
Department of General surgery, Fuwai Hospital, Beijing, China
27.1 General Considerations
Pulmonary atresia (PA) is a form of congenital pulmonary dysplasia in which the PV does not form properly; the duct of the RV and the pulmonary artery have an interruption in which there may be a residual ligament, or the artery may be totally separated. The range of pulmonary atresia may be limited or diffuse, and the portion of the atresia may be single or multiple. Generally, PA is classified according to two types: 1) PA with intact ventricular septum (PA-IVS) and 2) PA with VSD (PA-VSD) (Fig. 27.1c). The IVS type involves the arterial duct, accompanied by hypoplasia of the RV and the TV and may even include deformity of the coronary artery. The VSD type is characterized by underdevelopment of the RVOT accompanied by a large VSD and overriding of the aorta.
27.2 Embryology and Pathology
The PA-VSD shares the same basis of embryology with that of TOF, but with a different extent of stenosis of the pulmonary artery (Fig. 27.1e). The aortic and pulmonary arteries originate from the arterial stem, which comes from the far end of the bulbus cordis, and then the conotruncus rotates and becomes the aortic artery and the main pulmonary artery. However, if the conotruncus fails to rotate completely and the conotruncal septum fails to connect the ventricular septum, such that the ventricular septum with membranous septum and muscular septum are not closed simultaneously, a VSD results (Fig. 27.1f). However, if the muscular septum grows well, the whole septum should be intact. The forward malposition of the conotruncus may cause right ventricular infundibulum stenosis or atresia.
The PA-IVS is usually caused by a mild form of dysontogenesis of the main pulmonary artery, which may be accompanied by dysontogenesis of the RV.
In the case of the PA-VSD, the left and right pulmonary arteries mostly connect with each other and extend to the atretic main pulmonary artery. In some mild cases, there is some lumen in the root atresia, and the branches of the pulmonary artery grow well, so the arterial duct affords the blood supply. In severe cases, the pulmonary artery is long and narrow or agenesis, followed by stenosis of the left and right pulmonary arteries (Fig. 27.2g).
In addition to the arterial duct, the lateral branch between the aortic artery and the pulmonary artery is another source of the blood supply for the lung and often originates from the proximal end of the descending aorta; the branches may join with the artery of the lung lobe or the pulmonary arterioles. The lateral branch has some of the characteristics of muscular arteries, but the stenosis caused by the pressure from the descending aorta should not be ignored.
According to the levels of growth of the PA, with or without VSD, the source of the blood supply to the lung provides a means to classify the PAs. From the clinical perspective, we should emphasize four points: (1) whether there is remaining lumen in the far end of the root atresia or not; (2) the growth and connection of the left and right pulmonary arteries; (3) the main blood supply to the lung, the quantity and position of the atrial branch between the aortic artery and the pulmonary artery, and whether the blood comes totally from the lateral branch; and (4) the relationship between the VSD and the AV and the PV (Fig. 27.3).
Because of the separation between the pulmonary artery and the ventricle, the author separates the pulmonary atresia from the deformity of the connection between the ventricle and the artery (e.g., TGA).
27.3 Policy of Surgical Operation
27.3.1 Palliative Operation (Fig. 27.4)
When the blood supply is diminished, we preferentially increase the blood supply using procedures such as the improved Blalock-Taussig bypass or the Waterston bypass:
1.
Improved Blalock-Taussig bypass. A tunnel should be built from the right subclavian artery to the right pulmonary artery with an artificial shunt. According to the age and weight of the child, we choose different calibers of polytetrafluoroethylene (PTFE), usually 4–5 mm in diameter for an infant and 6 mm in diameter for children (Fig. 27.1a).
2.
Waterston anastomosis. If the pulmonary artery is wide enough, a side-to-side anastomosis can be executed between the ascending aorta and the main pulmonary artery.
3.
Bidirectional Glenn operation. The SVC is disconnected in the root, the proximal end to the heart is closed, and the far end to the right pulmonary artery is sutured. After 1–3 years, a second thoracotomy for shutting the VSD can be considered; if the case is accompanied with obstruction of the RVOT, reconstruction, including widening the outflow tract with a patch or replacing the old shunt from the RV to the pulmonary artery, can be considered.
27.3.2 One-Stage Radical Operation
The corrective surgery for PA-VSD involves building a tunnel from the RV to the pulmonary artery and repairing the VSD. In the case of main pulmonary atresia, one can adopt the pulmonary fusion, which involves building an outer tunnel between the RV and the lumen composed of the combination of the lateral branch between the aortic artery, the pulmonary artery, and the left and right pulmonary arteries. One-stage pulmonary fusion has become an important way to treat pulmonary atresia (Fig. 27.5).
A one-stage radical operation adopts deep hypothermic circulatory arrest (DHCA) or extracorporeal circulation. One should ligature the arterial duct before blocking the ascending aorta. The left and right pulmonary arteries should be dissociated; if the main pulmonary artery degenerates, the dissociation should be continued to the crotch. The left and right pulmonary arteries and lateral branches should be joined together, and a new lumen should be made by combining the RV through the outer tunnel. The VSD is repaired at last.
27.4 Specimen Demonstrations
27.4.1 Pulmonary Atresia, Ascending Aorta-Pulmonary Bypass
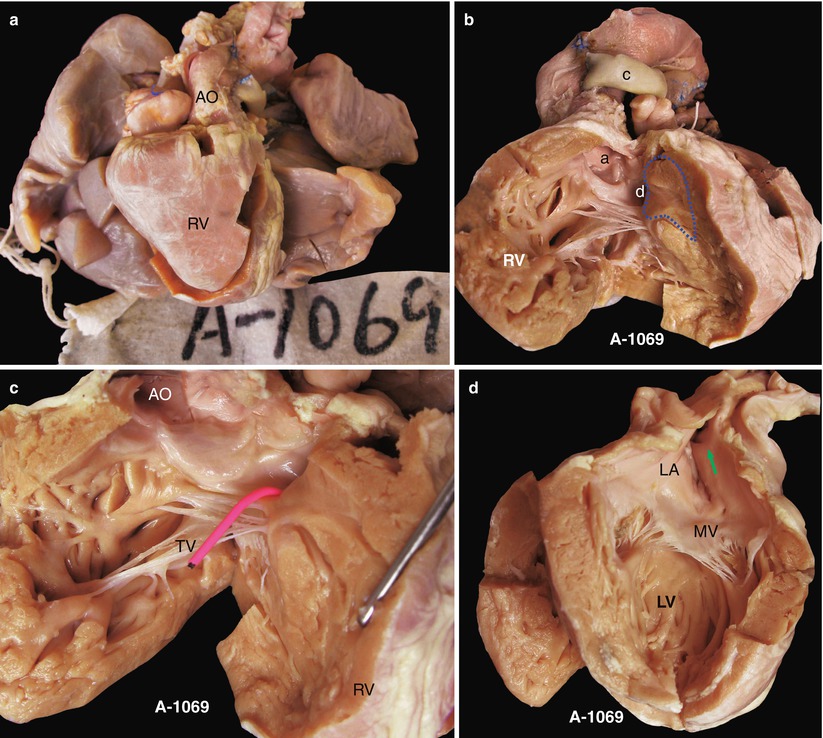
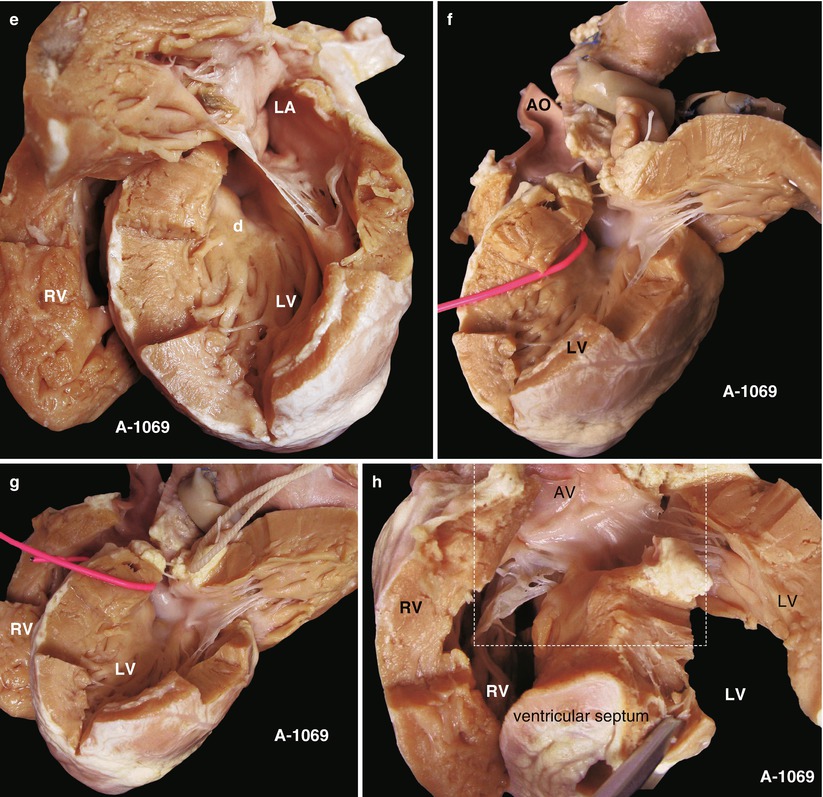
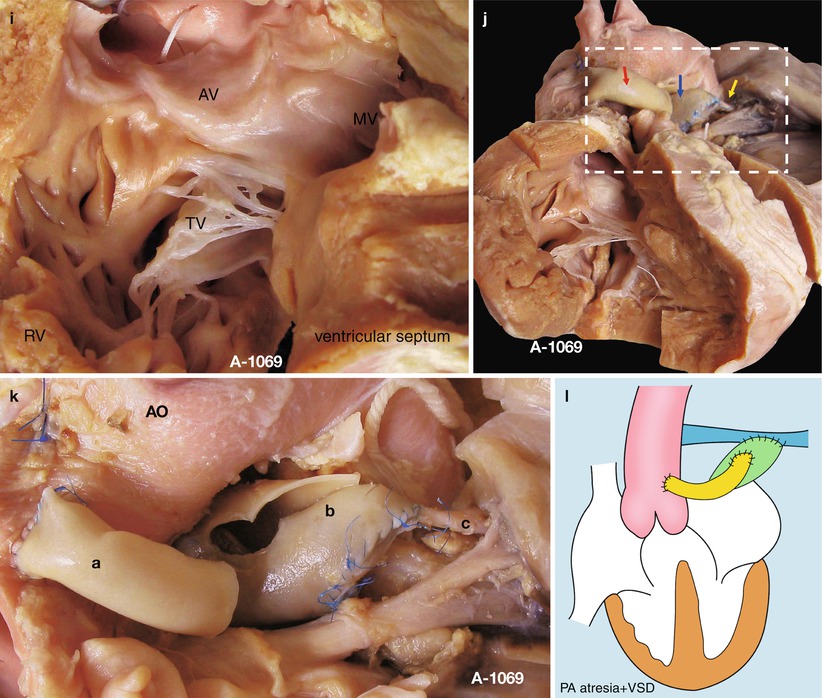
Fig. 27.1
(a) Specimen 1069. Appearance of the heart and lung in pulmonary atresia. The inflow and outflow tracts of the RV have been divided, the ascending aorta is thick and large but without the main pulmonary artery, and a shunt is connected with the left pulmonary artery in the left side of the ascending aorta, through which is a lumen. This case has accepted pulmonary fusion. AO ascending aorta. (b) Specimen 1069. Outflow tract of right ventricle. Figure shows that the AV (a) originates mostly from the R, and the VSD (d) is under the AV, without a PV. There is a thick parietal band hypertrophic supraventricular crest, and the wall of the RV is obviously thickening. Dotted line, thick parietal band; c artificial shunt. (c) Specimen 1069. VSD. The red probe is in the VSD; the AV mostly bestrides on the RV and extends to the TV. AO ascending aorta. (d) Specimen 1069. Left atrium slightly enlarged. The green arrow indicates the entrance of the pulmonary vein; the MV bestrides on the edge of VSD. LA left atrium, LV left ventricle. (e) Specimen 1069. The left atrium and the left ventricle. The VSD (d) can be seen through the left outflow tract under the MV; the depth of the wall of RV and LV is comparable. LA left atrium. (f) Specimen 1069. View of the left side. The red probe is through the VSD. A leaflet of the AV remains in the side of the LV and extends to the MV. There is no trace of PV in the left outflow tract. AO ascending aorta. (g) Specimen 1069. The fibrous connection of the aortic and mitral valves. Figure shows the VSD bestriding the AV. The red probe is through the VSD, and a fibrous connection of the AV and MV is seen. RV right ventricle, LV left ventricle. (h) Specimen 1069. The relationship of the AV, the MV, and the TV. The ventricular septum is cut up in the edge of the VSD in order to show the MV and the TV. This picture shows the fibrous connection of the leaflet of the AV and the MV and the TV. The dotted line indicates the point for observing. RV right ventricle, LV left ventricle. (i) Specimen 1069. The structure of Fig. 27.1h and the dotted line. The doorway of the AV is mostly in the RV, with only a leaflet of AV that bestrides the deficient ventricular septum; there is a fibrous connection of the AV and the MV and the TV. (j) Specimen 1069. The aorta-to-pulmonary artery bypass. The procedure divides the left and right pulmonary arteries (yellow arrow) and makes a tunnel with a piece of pericardium (blue arrow) so as to enlarge the pipeline of the pulmonary artery; the artificial shunt (red shunt) is sutured to the side wall of the aorta, and the artificial shunt is connected with the pericardial tunnel to finish the bypass. (k) Specimen 1069. Same as Fig. 27.1j, the figure shows the details of the bypass. a, the artificial shunt; b, pericardial tunnel; c, the left pulmonary artery. AO ascending aorta. (l) Specimen 1069. Sketch map of the operation. Attention should be given to the shunt from the aorta to the pulmonary artery; there is a fibrous connection of the AV and the MV and the TV
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


