51 Pulmonary and Tricuspid Valve Interventions
 Introduction
Introduction
Acquired pulmonary and tricuspid valve disease in the adult population is unusual and mostly relates to rarities such as carcinoid disease, rheumatic fever, and infective endocarditis, typically in the context of intravenous drug use. For those with congenital heart disease, however, dysfunction of these valves is both a primary component of many conditions and, in the case of the pulmonary valve, also a common consequence of several early repair strategies. With growing information regarding the harmful effects of chronic pulmonary regurgitation, surgical revision of the right ventricular outflow tract (RVOT) is now a commonly performed operation in this population, with some patients requiring several reoperations during a lifetime to maintain valvar function. Transcatheter pulmonary valve implantation (TPVI) was first proposed and tested experimentally by one of the authors in the year 2000.1 The procedure is now gaining widespread clinical acceptance, as its potential to reduce surgical reintervention in adults with repaired congenital heart disease is increasingly recognized. The Melody transcatheter valve now has both European and American regulatory approval, while other devices are under investigation for use in this position. Percutaneous tricuspid valve replacement, on the other hand, remains at a much earlier stage of development with initial clinical applications just beginning to emerge.2 In this chapter, we reflect on the progression of this revolutionary technology since the last edition of this textbook, discuss current indications and patient selection, report updated clinical results, and contemplate future directions.
 Transcatheter Pulmonary Valve Implantation
Transcatheter Pulmonary Valve Implantation
Background and Clinical Indications
Progress in surgery for congenital heart disease over the last 60 years has led to a considerable improvement in survival, with over 85% of babies now reaching adulthood.3 As a result, the prevalence of complex congenital heart disease in the adult population more than doubled between 1985 and the year 2000 and will continue to rise for the foreseeable future.4 Focus has therefore shifted toward the management of late morbidity in this growing population, with repeated surgery or catheter intervention often employed to treat various residual lesions or complications. Pulmonary regurgitation, which is common after transannular patch repair of tetralogy of Fallot, is a major cause of morbidity and may cause right ventricular dysfunction, impaired exercise capacity, and an increased risk of ventricular arrhythmia and sudden death.5–7 RVOT obstruction may also cause symptoms in patients with conduits or following the arterial switch operation.8–10 Surgical pulmonary valve replacement can halt and may reverse these detrimental outcomes.11–13 Severe pulmonary regurgitation with symptoms or reduced exercise tolerance is a class I indication for surgery. Valve replacement is also considered reasonable if severe pulmonary regurgitation is accompanied by moderate to severe right ventricular dysfunction or enlargement, symptomatic or sustained atrial and/or ventricular arrhythmias, or moderate to severe tricuspid regurgitation. For those with RVOT obstruction, surgery is considered in the presence of a peak Doppler gradient >50 mm Hg, a right-to-left ventricular pressure ratio >0.7, progressive and/or severe right ventricular dilation with dysfunction, or if there are associated lesions requiring surgical repair. In conduit dysfunction, percutaneous intervention may be useful where the diameter narrowing of the prosthesis is >50%.14 It has been suggested, however, that intervention is being performed too late, as the ability of the right ventricle to remodel following surgery may be limited.15 Implanted biological valves, however, have a limited life span, and the desire to avoid progressive right ventricular dysfunction has been moderated by the risks associated with redo surgery and cardiopulmonary bypass.16,17 Additionally, placement of bare stents for RVOT obstruction has been complicated by inevitable pulmonary regurgitation.18 TPVI is a transcatheter approach that can treat both pulmonary regurgitation and stenosis in patients with suitable RVOT anatomy. Traditional criteria for surgery have provided the baseline clinical indications for this new technique. Inclusion criteria in the U.S. Melody Trans-catheter Pulmonary Valve trial required a mean Doppler gradient ≥35 mm Hg or at least moderate pulmonary regurgitation in symptomatic patients or a mean Doppler gradient ≥40 mmHg or severe pulmonary regurgitation with an associated tricuspid valve annulus z-score ≥2 or right ventricular fractional shortening <40% in asymptomatic patients.19 Because of technical feasibility relating to the dimensions of the delivery system, the procedure is also restricted to patients over the age of 5 years with a weight above 30 kg. Once established, the less invasive nature of TPVI will support the current inclination for earlier intervention and offer a treatment option to those who are not surgical candidates.20 Importantly, TPVI does not affect subsequent suitability for surgery. Careful investigation will be required to redefine the indications clearly and determine the optimal timing for treatment in this growing patient population. In some rare cases of acquired pulmonary valvular disease (e.g., carcinoid disease), TPVI may also be considered as a treatment option.
The Device
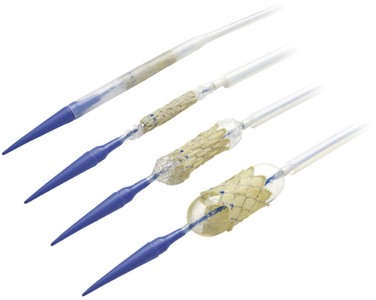
The Medtronic transcatheter pulmonary valve (Melody, Medtronic, Minneapolis, MN) is composed of a segment of bovine jugular vein with a thinned down wall and a central valve (Fig. 51-1). The vein is sutured inside an expanded platinum-iridium stent (length, 28 mm; diameter, 18 mm) that can be crimped to a size of 6 mm and reexpanded up to 22 mm. The current stent design, which has an eight-crown zig pattern with six segments along its length, is reinforced at each strut intersection with gold weld. The venous segment is attached to the stent by continuous 5-0 polypropylene sutures around the entire circumference at the inflow and outflow and also discretely at each strut intersection. The suture is clear for all points except the outflow line, which is blue to signify the outflow end of the device. The venous segment is fixed in a buffered glutaraldehyde solution in a concentration low enough to preserve the flexibility of the venous valve leaflets. A final sterilization step is performed on the combined device using a proprietary sterilant containing glutaraldehyde and isopropyl alcohol, in which it is then packaged.
The Delivery System
The delivery system, also manufactured by Medtronic (Ensemble, Medtronic, Minneapolis, MN), comprises a balloon in balloon (BiB) deployment design at its distal end onto which the valved stent is front-loaded and crimped (Fig. 51-2). The system is available with three outer balloon diameters: 18, 20, and 22 mm. The tip of the system is blue to correspond with the outflow suture of the device and encourage correct orientation. The body of the system is composed of a one-piece Teflon sheath containing a braided wire-reinforced elastomer lumen. This design minimizes the risk of kinking while optimizing flexibility and retaining the necessary pushability required for the procedure. There is a retractable sheath that covers the stented valve during delivery and is pulled back just prior to deployment. Contrast can be delivered via the retracted sheath from a side port to confirm positioning of the device prior to deployment. Proximally, there are three ports, one for the guidewire (green), one to deploy the inner balloon (indigo), and one to deploy the outer balloon (orange).
Animal Studies
The feasibility of TPVI was first demonstrated in lambs using a device that combined commercially available products (Contegra, Medtronic, Minneapolis, MN) and CP (Cheatham Platinum) stent (NuMed Inc., Hopkinton, NY) and was a precursor of that described above.21,22 Bench testing had already confirmed that crimping and reexpansion of the device by balloon catheter would not affect valvar competence. Devices were delivered from a right internal jugular approach under fluoroscopic guidance. The valved stents were deployed in the native pulmonary artery of 7 of 11 lambs, with the procedure failing in 4 owing to an inability to cross the tricuspid valve. In humans, the femoral vessel, which is relatively larger, can be utilized and promotes a straighter catheter course, thus overcoming this technical difficulty. Of 7 valved stents, 5 were implanted in an optimal position impinging on the function of the native valve. No complications occurred during the procedure or follow-up. Two of the animals developed a mild fever, but this disappeared in 48 hours without intervention. During the subsequent 2 months, all animals were asymptomatic and nearly doubled their body weight. At the end of the protocol, hemodynamic evaluation showed normal pulmonary pressures in all lambs. One stent was mildly stenotic with a gradient of 15 mm Hg across it. At autopsy, fibrosis of the valve leaflets occurred in the two devices that had not been implanted in the desired position. Subsequently, valved stent designs have been implanted in the pulmonary position in other trials employing animal models, with positive results.23–25 Histological investigation has also shown that calcification of valved stents in this position occurs in the wall portions without affecting the cusps and that cardiac structures in the vicinity have normal histology without inflammation.26
Clinical Studies
The first human application of transcatheter pulmonary valve implantation was reported in the year 2000. A 12-year-old boy with an original diagnosis of pulmonary atresia and ventricular septal defect underwent TPVI to treat stenosis and insufficiency of an 18-mm Carpentier-Edwards conduit that had been placed between the right ventricle and pulmonary artery when he was 4.27 The procedure was uncomplicated and resulted in complete relief of the insufficiency and partial relief of the stenosis. Since then, the procedure has been performed in over 1,200 patients and more than 90 centers worldwide. The London/Paris experience (155 patients) and the U.S. experience (136 patients) currently comprise the largest reported series in the literature.28,29 The results are described below.
The Procedure
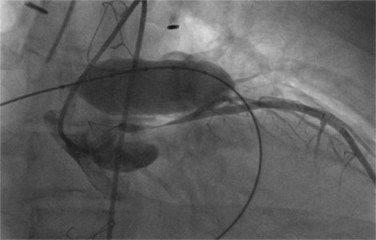
Under general anesthesia with invasive blood pressure monitoring, TPVI is performed predominantly via a right femoral venous approach. A full aseptic technique to surgical standards is used and a single dose of broad-spectrum intravenous antibiotics is given for endocarditis prophylaxis. Heparin is administered routinely at the beginning of the procedure and repeated hourly thereafter as required to maintain an activated clotting time >250 seconds. Right heart catheterization is performed according to standard techniques to assess pressures and saturations. Routinely, measurements are made in the right ventricle, pulmonary artery, and aorta with additional measurements (e.g., in the branch pulmonary arteries) made as appropriate. A stiff guidewire (0.035 Amplatz Ultrastiff, Cook Inc., Bloomington, IN) is then positioned into a distal branch pulmonary artery to provide an anchor from which to advance the delivery system. First, biplane angiography is performed using a Multi-Track catheter (NuMed Inc., Hopkinton, NY) with the tip placed just beyond the expected position of the pulmonary valve to allow assessment of the proposed site for device implantation and quantification of pulmonary regurgitation. Angiography is also performed in the aortic root. If there is suspicion that a coronary artery is at risk of compression from valve implantation, coronary angiography is performed with an angioplasty balloon (18–20 mm Mullins balloon, NuMed Inc., Hopkinton, NY) simultaneously inflated in the conduit. If there is a risk of coronary compression, implantation should not be attempted and the patient referred for surgery (Fig. 51-3). Conduit predilation should also be performed using a balloon 2 mm larger than the narrowest diameter of the conduit and less than 110% the original conduit diameter (PTS sizing balloon, NuMed Inc., Hopkinton, NY). If the balloon waist measures between 14 and 20 mm on subsequent low-pressure (≤8 atm) balloon sizing, the conduit can be considered anatomically suitable and the procedure can continue. To minimize the risk of conduit rupture, the diameter of the predilation balloon or the delivery system balloon used should not exceed 110% of the nominal diameter (original implant size) of the conduit. Concurrently, the valved stent is prepared in three sequential saline baths (5 minutes in each) to wash off the glutaraldehyde in which it is stored. The size of the valved stent is reduced by crimping it on mandrels of increasingly smaller sizes prior to front loading onto the delivery system. It is recommended to use a 2.5-mL syringe for crimping to an intermediate size prior to final crimping onto the balloon catheter. The blue stitching on the distal portion of the device is matched to the blue portion of the delivery system and verified by an independent observer to guarantee correct orientation. Further hand crimping of the device onto the balloon is performed, following which the sheath is retracted over the device while a saline flush is administered via the side port to exclude air bubbles from the system. Following removal of the Multi-Track catheter, the femoral vein is dilated to 24 Fr and the front-loaded delivery system is advanced into the RVOT under fluoroscopic guidance. The sheath is then retracted from the valved stent and contrast is injected via the side port to confirm position. Partial deployment is achieved by hand inflation of the inner balloon and, after final confirmation of the position, the outer balloon is also hand-inflated to complete deployment. The balloons are then deflated and the delivery system is withdrawn. Repeat angiography and pressure measurements are made to confirm a positive outcome. Post-dilation of the valve may be performed at the discretion of the operator.
Modification of the Technique
The nature and position of right ventricular to pulmonary artery conduits is heterogeneous; cannulation with a large delivery system can therefore be challenging. Predilation (Mullins balloon and indeflator, NuMed Inc., Hopkinton, NY) and in some cases bare stenting (Max LD, EV3, Plymouth, MN) conduits that are heavily calcified or tortuous can facilitate passage of the system in addition to optimizing the final result. This may also have the additional benefit of reducing the risk of future stent fractures.30 Further maneuvers that can be used to advance the delivery system when it is at the entrance to the conduit include looping the system within the right atrium, partial retraction of the sheath, and repositioning of the guidewire in the contralateral branch pulmonary artery. The first two actions generate a forward force often overcoming any resistance the system is experiencing and aid passage into the conduit. Once deflated, the delivery system is withdrawn. If further dilation of the valved stent is required, this is performed using a high-pressure Mullins balloon and indeflator (NuMed Inc., Hopkinton, NY). Care is taken not to dilate conduits beyond their original documented size to minimize the risk of rupture. Postdilation of the device has not been observed to cause any damage to valve leaflets or affect valve competency. Although the preferred approach is via the right femoral vein, mainly for practical and logistical reasons, successful valve implantation has also been achieved via left femoral, right and left internal jugular, and left subclavian veins. A transhepatic route is neither used nor recommended in view of the size of the delivery system.
Results
Hemodynamics
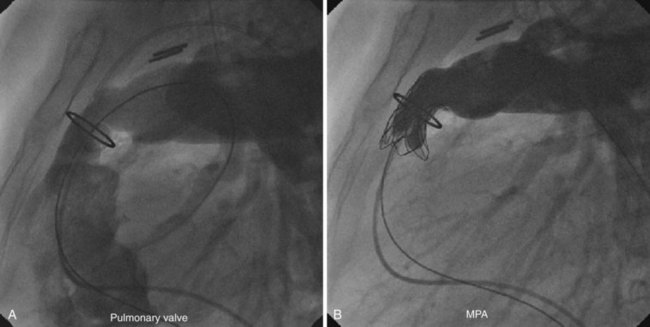
Following TPVI, angiography showed a significant improvement in regurgitation, with no one in either cohort having more than trivial to mild regurgitation (Fig. 51-4). RVOT gradient and RV pressures fell significantly in patients with stenotic lesions as well as those with predominant regurgitation, albeit to a lesser extent. In this group, pulmonary artery diastolic pressure increased, reflecting the restoration of a competent pulmonary valve. All patients experienced a small rise in systemic pressure, which might reflect improved cardiac output but could also indicate lightening of the anaesthesia toward the end of the procedure. Subsequent investigation has suggested that different patterns of hemodynamic change can be expected depending on the nature of the RVOT dysfunction being treated.31,32