Viola Vaccarino, J. Douglas Bremner
Psychiatric and Behavioral Aspects of Cardiovascular Disease
Recognition of the importance of psychological stress and emotional factors as potentially modifiable risk factors for cardiovascular disease (CVD) is growing. Psychological stress potentially contributes to CVD at several stages of the disease process, from influencing risk factors for CVD, to affecting the development of atherosclerosis and subclinical CVD, to impairing recovery, prognosis, and quality of life of patients who have survived an acute coronary syndrome.1
Stress Response and Cardiovascular Disease
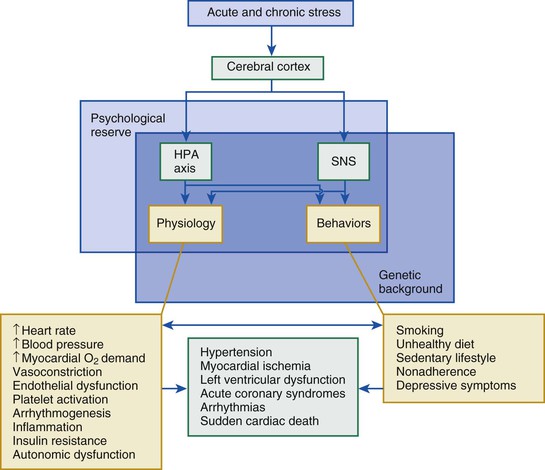
The stress response, an adaptive physiologic mechanism that allows the organism to respond to life-threatening situations, results in stimulation of the sympathoadrenal system and the hypothalamic-pituitary-adrenal (HPA) axis with release of cortisol and catecholamines (see Chapter 89). This concept has evolved from a simple model of a “fight or flight” response to threat to better understanding of the more complex interactions of the physical and social environment with the brain and the body involving the neuroendocrine, autonomic, metabolic, and immune systems.2 Acute activation of these systems mobilizes energy and prepares the individual for adequate coping with stressors. With chronic exposure to stress, however, repeated attempts at adaptation may result in pathologic perturbation with adverse consequences on hemodynamics, metabolism, inflammation, and immune function.
Psychological factors have been implicated both as triggers of acute coronary events and as promoters of the atherosclerotic process. A number of possible underlying biologic mechanisms have been proposed, including among others, a repeated or sustained increase in blood pressure and heart rate, insulin resistance and other metabolic abnormalities, systemic vascular resistance, autonomic dysregulation, ventricular arrhythmias, and dysregulation of the inflammatory and immune systems (Fig. 86-1). In addition to biologic mechanisms, lifestyle plays an important role inasmuch as stress, especially in the presence of stress-related disorders such as posttraumatic stress disorder (PTSD) and depression, increases the risk for adverse behavior such as smoking, drug and alcohol abuse, poor eating habits, and lack of adherence to preventive measures and treatment recommendations. However, individual responses to stress vary. Such responses can be modulated by genetic factors and by buffering psychosocial resources, such as social support, optimism, and other personality traits, which represent what is also known as resilience or “psychological reserve capacity.”
Methodologic Issues
Animal models, particularly nonhuman primates, have provided experimental evidence of the adverse cardiovascular effects of chronic stress.3 In humans, however, demonstration of a causal link between stress and CVD has been difficult to prove. From a methodologic standpoint, two major research strategies have been used to study the role of psychological factors on risk for CVD in human populations. The first includes standard epidemiologic methods to examine the relationship between exposure to stressors or other psychological factors and CVD endpoints. The second approach, which provides a more direct mechanistic insight, is to measure cardiovascular responses to a standardized “mental stress test” in the laboratory. Although the effects of experimentally induced stress in the laboratory on the cardiovascular system are well documented, determination of the impact of naturally occurring acute or chronic stressors on CVD by traditional epidemiologic methods has been more challenging to demonstrate. One problem is the definition of exposure. In the field of epidemiology, under the general term of “psychosocial stress,” investigators have included a number of related but not necessarily synonymous constructs that encompass environmental exposures of different sources, intensities, and durations (from acute stressors; to long-term job, financial, or family difficulties; to minor everyday hassles), as well as individuals’ subjective responses to stressors and emotional states. In the mental health fields of psychology and psychiatry, investigators have focused on psychiatric diagnoses of stress-related mental disorders and used a definition of traumatic stress, in its current iteration, as a threat to life or self-integrity associated with intense fear, horror, or helplessness. All these exposures are by definition self-reported and typically retrospective in nature, thus further challenging their accuracy. A related problem is the lack of standardized measures to consistently define and quantify the type and severity of psychological stress. Despite these methodologic challenges, psychosocial factors are increasingly being recognized as important and potentially modifiable risk factors and prognostic indicators for CVD.
Objectives
The goal of this chapter is to review the epidemiologic and pathophysiologic evidence linking psychological factors to CVD and discuss their clinical relevance and management in the current practice of cardiology. From a classification standpoint, psychological/psychiatric risk factors for CVD can be grouped into four main categories: (1) acute stressful triggers of cardiac events; (2) chronic stressors, including among others, work stress, low socioeconomic status (SES), marital and caregiving stress, and lack of social support; (3) personality traits; and (4) psychiatric diagnoses, including depression and anxiety disorders.
Acute Stress
Stressful and Emotional Triggers of Acute Coronary Events
Many studies, albeit not all, have demonstrated an increase in hospital admissions for acute coronary syndromes after emotionally stressful events such as natural and industrial disasters, terrorist attacks, and sporting events (see Chapter 53).4 During the 1994 Northridge earthquake in the Los Angeles area, hospital admissions for acute myocardial infarction increased 35% in the week after the earthquake relative to the week before. Based on coroners’ records, sudden cardiac death increased from an average of 4.6 events per day in the week before the earthquake to 24 events on the day of the earthquake and then fell to 2.7 per day in the next 6 days. Only three of these cases were associated with unusual physical exertion. Coronary deaths tended to be clustered around the epicenter of the earthquake, and there was no increase in non–coronary-related deaths. Although similar data have been reported after other earthquakes, the results are not entirely consistent, possibly because of variations in the timing and season of the earthquakes. Earthquakes occurring in winter and early in the morning are more closely related to cardiac events.
War and terrorist attacks have also been associated with acute coronary events. During the initial phases of the Gulf War in the Tel Aviv area in 1991, for example, the incidence of acute myocardial infarction and sudden death increased. By contrast, the World Trade Center terrorist attack in New York City on September 11, 2001, was not linked to an increase in cardiac events immediately following the attack. However, the incidence of cardiovascular ailments diagnosed by physicians increased by more than 50% in the following 3 years, thus suggesting a more chronic impact of the attack rather than an acute triggering effect. In addition, ventricular arrhythmias increased by more than twofold in patients with implantable cardioverter-defibrillators (ICDs; see Chapter 36), but only during a period from 3 to 30 days after the event. It is possible that the 9/11 attack, which was covered extensively on television, did not cause generalized acute stress as did other events that posed a direct threat to personal safety but had a more subacute and protracted effect on the population.
The impact of major sports matches on cardiac events among spectators is also well documented. Increases in cardiac events and death are more pronounced in the 2 hours after the start of the matches and affect men more than women.5
A limitation of these ecologic studies is lack of information on the individual circumstances surrounding cardiac events, which makes it difficult to rule out alternative explanations. Apart from emotional stress, cardiac events could be triggered by physical exertion (such as running away) or dust and other environmental pollutants. In this respect, studies of emotional triggers at the individual level, in which patients are asked about their experiences before the onset of symptoms, should provide more information. On the other hand, such studies can include only survivors of the acute coronary event and must rely on retrospective assessments of the stressor, which may be affected by recall bias. This latter limitation is mitigated by use of the case crossover design, whereby the risk period before the index cardiac event is compared with a control period in the same individual. By using this method, studies have linked intense episodes of anger, acute work-related stressors, or acute sadness and bereavement to coronary events.4 In the Determinants of Myocardial Infarction Onset study, 2.4% of patients reported being very angry or furious in the 2 hours before acute myocardial infarction (see Chapter 51). When compared with a matched control period 24 hours earlier, the odds of acute myocardial infarction developing following acute anger were increased fourfold. In the SHEEP study, patients who reported a sudden, short-term increase in workload, such as a high-pressure deadline, exhibited a sixfold increase in the odds of myocardial infarction developing during the next 24 hours relative to a control period before the infarction. Acute depressed mood was associated with a 2.5-fold higher odds of acute coronary syndromes in comparison to a control period 24 hours earlier in the same individuals.6 Exposure to heavy traffic has also been related to the risk for myocardial infarction, and the time that subjects spend in cars or public transportation is directly related to the risk; in addition to stress, however, pollution and noise may contribute to this effect. Finally, stressful life events have been linked to acute myocardial stunning in susceptible individuals with severe, reversible left ventricular dysfunction. This syndrome is known as takotsubo cardiomyopathy or transient apical ballooning syndrome. The patients, almost all women, show exaggerated stimulation of the sympathetic nervous system as indicated by markedly elevated plasma catecholamine levels.
Potential Mechanisms
A key pathophysiologic event underlying acute coronary events is the progression from a stable plaque to a “vulnerable” plaque. Despite the lack of direct evidence that acute psychological stress causes rupture or erosion of atherosclerotic plaque, acute episodes of stress or intense emotions may trigger acute coronary events in susceptible individuals by affecting plaque stability and disruption through hemodynamic activation (increases in blood pressure and heart rate), increased systemic vascular resistance, coronary vasoconstriction, inflammation, and prothrombotic effects, among others. Triggering usually takes place against a background of advanced atherosclerosis; thus it is considered rare in people without underlying coronary artery disease.4
Insights from Studies of Mental Stress
A useful method of assessing the effects of stress and emotion on cardiac function is to measure transient cardiovascular responses to a standardized psychological stress challenge in the laboratory, also known as a “mental stress test,” by using techniques such as mental arithmetic, color naming, public speeches, anger recall, and similar tasks (see Chapter 49). This methodology has the advantage of direct experimental manipulation in which potential confounding factors can be controlled or eliminated and causal factors and their mechanisms directly investigated. However, this approach is necessarily limited to short-term responses to acute stress artificially induced in the laboratory and therefore may lack practical significance. To address this issue, longitudinal studies have investigated the link between mental stress–induced cardiovascular responses and future CVD events. A recent systematic review of this literature showed that greater cardiovascular reactivity to mental stress (defined mostly as acute changes in blood pressure and heart rate) and poor recovery from stress (defined as sustained cardiovascular activation above baseline levels during the post-task period) are associated longitudinally with cardiovascular outcomes, including elevations in blood pressure and CVD events, whereas evidence of an association with atherosclerosis endpoints such as carotid intima-media thickness and coronary artery calcifications is more limited.7 Cortisol and catecholamine responses to mental stress have also been related to future hypertension and other CVD endpoints.
Mental Stress–Induced Myocardial Ischemia
In addition to cardiovascular reactivity, an important phenomenon that has been studied in conjunction with mental stress in cardiac patients is mental stress–induced myocardial ischemia. The latter is analogous to ischemia induced by exercise or pharmacologic stress, except that the stimulus is psychological, and it has similar prognostic significance.8 It can be induced in one third to two thirds of patients with coronary heart disease. It is typically painless and occurs at lower levels of oxygen demand than does ischemia secondary to physical exertion. In addition, ischemia induced by mental stress is not generally related to the severity of coronary artery disease. Ischemia may develop with mental stress, but not with exercise or pharmacologic stress, although results vary. Mental stress–induced (but not exercise-induced) myocardial ischemia correlates with ischemia measured in daily life ambulatory monitoring. Thus, mental stress testing could theoretically provide a means for identification of patients vulnerable to myocardial ischemia in everyday life.
Results published to date have indicated that mental stress–induced ischemia is a predictor of a poor prognosis. Several patient series with a follow-up period ranging from 1 to 5 years have found substantial increases, between 70% and threefold, in cardiovascular events, revascularization procedures, and death when comparing cardiac patients with mental stress–induced ischemia with those without, independent of the severity of coronary disease and risk factors for CVD.8 Although the samples of patients observed longitudinally to date are relatively small, the overall evidence indicates that myocardial ischemia in response to a standardized mental stress test is at least as prognostically important as exercise-induced ischemia, if not more so.
The hemodynamic responses underlying ischemia triggered by psychological stress are different from those underlying exercise-induced stress. Mental stress–induced ischemia occurs at a lower rate-pressure product than does exercise-induced ischemia in the same patients, although the hemodynamic response tends to be larger in patients with mental stress–induced ischemia than in patients who do not become ischemic. Patients in whom ischemia develops in response to mental stress have an increase in systemic vascular resistance, thus suggesting a rise in afterload caused by peripheral vasoconstriction. By contrast, systemic vascular resistance is decreased by exercise. These effects may be secondary to centrally mediated neurogenic peripheral vasoconstriction; in fact, plasma catecholamine levels increase rapidly with mental stress and correlate with hemodynamic changes.9
Abnormal coronary vasoconstriction may develop in some patients with coronary artery disease during mental stress, particularly at points of stenosis, which may cause myocardial ischemia. This effect correlates with the endothelium-dependent response to an infusion of acetylcholine and suggests coronary endothelial dysfunction. Because such vasoconstriction can be reversed by alpha-adrenergic blockade (via intracoronary administration of phentolamine), the sympathetic nervous system appears to play a role.8,9
Mental Stress and Cardiac Electrophysiology
Acute mental stress can also induce cardiac electrical instability, including an increase in T wave alternans and other measures of abnormal cardiac repolarization that have been related to arrhythmogenesis and sudden cardiac death (see Chapter 37).10
Autonomic dysfunction, along with its effects on cardiac electrophysiology, is another process probably underlying the acute adverse effects of stress on the heart (see Chapter 89). Both sympathetic activation and parasympathetic withdrawal can stimulate arrhythmias and lower the threshold for ventricular fibrillation. Heart rate variability, a measure of the beat-to-beat changes in heart rate as the heart responds to internal and external stimuli, is an accepted noninvasive measure of overall cardiac autonomic function. Reduced heart rate variability predicts coronary heart disease in population studies, as well as mortality, particularly sudden death, in patients following acute myocardial infarction.11 Heart rate variability is reduced during acute mental stress in the laboratory and was found to be decreased during major disasters, such as earthquakes or terrorist attacks, in studies of patients who were undergoing ambulatory electrocardiographic monitoring at the time of the event.4 These mechanisms may underlie the connection described between acute stress and life-threatening cardiac arrhythmias and sudden cardiac death.
Mental Stress, Inflammation, and Immunity
The inflammatory and immune pathways are critical to atherogenesis, plaque progression, and thrombus formation. It is well established that the immune system responds acutely to psychological stress. Noradrenaline-dependent adrenergic stimulation as a result of stress-induced activation of the sympathetic nervous system activates the nuclear transcription factor kappa B (NF-κB) in circulating monocytes, which results in initiation of the inflammatory cascade. Parasympathetic stimulation has the reverse effect: inhibition of NF-κB activation. Consistent with these biologic effects, mental stress triggers robust increases in circulating inflammatory biomarkers, in particular, interleukin-6 and interleukin-1beta.12 Impaired poststress cardiovascular recovery after mental stress is also associated with sustained inflammatory and hemostatic responses. However, little prospective information is presently available to link inflammatory responses to an acute stress challenge with future cardiovascular endpoints.
Acute Stress and Cardiovascular Disease: Clinical Implications
The clinical significance of acute emotional triggers of cardiac events has not been clearly established. Although the relative risk associated with acute stress is substantial, the absolute risk is smaller given that these events are relatively uncommon. Accordingly, the population attributable risk (i.e., the reduction in disease that would be observed if the risk factor were eliminated entirely) is also not large (≈4%), but it is fairly similar to that of other acute triggers of coronary events such as physical exertion, heavy traffic, or excessive alcohol consumption.13 Although it has been argued that programs to increase awareness of psychological triggers among clinicians and the public would be beneficial, such programs in general lack evaluation. Whether therapies for prevention of CVD, such as aspirin, beta blockers, statins, and angiotensin-converting enzyme inhibitors, also protect against the harmful effects of emotional triggers is similarly not known.
Chronic Stress
Common chronic, or long-term, stressors in the general population include factors such as work-related stressors, financial difficulties or low SES, marital/caregiving stress, and low social support. Each of these factors has been evaluated extensively in relation to cardiovascular risk. A potential problem with this literature is that many of these exposures may be correlated with each other over the lifetime whereas individual studies have typically examined single factors without considering cumulative stress exposure from multiple sources. Although a few studies have included more general measures of perceived stress, they were not often specific enough to measure objective external exposure to stressors and are likely to be influenced by individual characteristics such as personality traits and emotional problems.
Work-Related Stress
Work stress has been studied extensively for its potential adverse cardiovascular effects. Two dominant models of work stress that have been associated with risk for CVD include the “job strain” model developed by Karasek and Theorell and the “effort-reward imbalance” model by Siegrist.14 The job strain model postulates that high work demands in combination with low control produce stress because workers in low-control jobs cannot moderate work pressure by organizing their time or by other means. The highest risk applies to situations of high demand, low control, and low social support at work. According to the effort-reward imbalance model, stress derives from a mismatch between high workload, such as long working hours, and low payback in terms of income, job security, job status, or other forms of recognition. Other less well-studied sources of stress in the workplace include unfair treatment and other forms of organizational injustice, job insecurity, and conflicts with coworkers. Recent systematic reviews have found a significant increase in the risk for CVD in various work stress models.14,15 In these pooled analyses, job strain was associated with a 40% increased risk for CVD, whereas imbalance between effort and reward was associated with a 60% increased risk for CVD.
In general, study results linking job stress to CVD have been stronger for men than for women. It is possible that stress in other life domains, such as family and social relationships, may be more important for women than job-related stress.16 It should be noted, however, that few studies are available on female samples. In addition, because many study cohorts have included working populations, they may have been too young to capture cardiovascular events among women. Finally, these studies have not typically included information on part-time work, which may be more frequent in women.
Most studies of work stress and CVD have examined initially healthy populations. Studies of patients with established coronary disease are fewer. In a Canadian study, job strain was associated with a twofold increase in risk for recurrent events among patients returning to work after a myocardial infarction.17 Similarly, in a Swedish cohort, a combined outcome of cardiac death and recurrent myocardial infarction was 70% more frequent in patients with high job strain than in those with low job strain.18 Because most studies have included predominantly male worker populations, data specific to women with CVD are limited. However, as for populations initially free of CVD, results generally appear weaker in women, even when women who work full-time outside the home were considered.
Marital and Caregiving Stress
Although work stress has been studied primarily in men, marital and caregiving stress has been studied primarily in women. In the Stockholm Female Coronary Risk Study, women who suffered a myocardial infarction and reported marital stress had an almost threefold higher risk for recurrent cardiac events than did women with less marital stress after adjusting for other risk factors.16 However, few studies are available on this dimension of chronic stress, and data on male populations are limited.
Caregiving for an ill family member is common, with approximately 12% of Americans older than 45 years reporting caregiving responsibilities. High caregiving stress has been associated with a variety of poor physical and psychological outcomes, including higher risk for CVD and mortality. In the Nurses’ Health Study, caregiving for an ill or disabled spouse was associated with an almost twofold increased risk for coronary events after adjusting for other risk factors. Similarly, in the Caregiver Health Effects Study, caregiving was associated with a 63% higher adjusted risk for mortality. In the REGARDS (REasons for Geographic and Racial Differences in Stroke) study, high caregiving strain was associated with a 23% higher risk for stroke, an effect that was larger in black American men.19 The adverse effects of caregiving, however, primarily apply only to caregivers who report psychological strain or distress; caregivers not experiencing strain do not have elevated risk.
Low Socioeconomic Status
SES is generally defined by one or a combination of interrelated factors such as occupational status, economic resources, education, and social class. Area-based SES measures, including census blocks or zip codes and other composite measures of neighborhood impoverishment, are increasingly becoming popular as SES measures because they correlate well with individual SES measures and can be used when the latter are not available.
The existence of a social gradient in health and disease has long been recognized. Beginning many decades ago, the Whitehall Study of British Civil Servants reported that even among people who have access to health care and are not poor, there is a social gradient in mortality and morbidity, including CVD, from the bottom to the top of society.20 Such results have been confirmed in many other contexts, including the United States.
Even in patients with suspected or confirmed CVD, disparities in outcome throughout the SES spectrum exist. In a U.S. study of more than 30,000 patients referred for cardiac stress testing, lower SES was associated with higher mortality, impaired exercise capacity, and abnormal heart rate recovery.21 Similarly, in a Canadian population with universal access to health care, lower household income correlated with significantly higher severity of coronary atherosclerosis in diabetic patients referred for cardiac catheterization.22 Low SES is accompanied by poorer health habits and more unfavorable standard risk factors for CVD, such as hypertension, obesity, smoking, sedentary lifestyle, and unhealthy diet, which, however, only partially account for the CVD gradient attributable to social class.23 Many adverse psychosocial characteristics are also related to lower SES, including financial hardship, poorer housing, neighborhood status, social discrimination and isolation, depression, and adverse working conditions. Thus low SES can be viewed as a composite of chronic stressors that may result in adverse behavioral and physiological consequences. Lower access to preventive medical care may also play a role in societies, such as the United States, that do not have universal health care systems.
HPA axis and autonomic dysfunction is observed as SES declines, which may increase the risk for central obesity and metabolic risk factors. The Whitehall II study, for example, described a close relationship between lower social position and increased prevalence of the components of metabolic syndrome, an association that was only partially explained by health behavior.23 Disturbances in neuroendocrine and cardiac autonomic activity, compatible with activation of neuroendocrine stress axes, were also noted in subjects with metabolic syndrome and those with lower SES status. Notably, psychosocial factors (SES- and job-related stress) explained a large proportion of the association between adrenal/autonomic disturbances and metabolic syndrome.
Adverse Childhood Experiences
Adverse childhood experiences, sometimes referred to as “early life stress,” are commonly defined as various forms of maltreatment in childhood, such as verbal, physical, and sexual abuse. Some definitions also include indicators of family dysfunction, such as domestic violence or the presence of family members who are drug addicted, incarcerated, or mentally ill. Adverse childhood experiences are quite prevalent in the general population. In a recent national survey of adults, approximately one quarter (26%) reported verbal abuse in childhood; 15%, physical abuse; and 12%, sexual abuse.24 Adverse childhood experiences have been linked to a range of adverse health outcomes in adulthood, including substance abuse, depression, PTSD, CVD, and premature mortality.25 A recent meta-analysis confirmed a link between childhood maltreatment and a number of medical outcomes in adulthood, including CVD.26 Despite heterogeneity of effects across studies, the association is seen both when abuse is measured by self-report and when it is measured objectively.
In terms of mechanisms, although the literature is limited by many studies being cross-sectional or retrospective, the bulk of the evidence links adverse childhood experiences to enduring changes in the nervous, endocrine, and immune systems.25 A history of childhood maltreatment has been associated with smaller volumes of the prefrontal cortex and the hippocampus, greater activation of the HPA axis during stress, and elevated inflammation.27 These changes persist in adulthood, thus providing evidence for an enduring effect of early life stress on physical health. For example, a prospective study linked childhood maltreatment to adult inflammation.28 Maltreated children showed higher inflammation 20 years later, which persisted after accounting for other childhood exposure and health behavior.
Social Isolation, Loneliness, and Lack of Support
Social isolation and loneliness appear to be increasingly common; for example, between 1985 and 2004, the number of people with no one to discuss important matters tripled to 25%.29 The size, quality, and perceived adequacy of a person’s social contacts have all been related to CVD and total mortality. Social relationships may improve health in a variety of ways, including provision of instrumental and emotional support and encouragement toward a healthy lifestyle and health care seeking. Emotional support may also buffer the adverse effects of psychological stressors. Reverse causation is possible as well, however, in that individuals who are ill or otherwise at risk may be less socially engaged.
Despite scientific interest in these constructs, there is little theoretical integration of their various dimensions and little consensus on measurements and mechanisms. Although a number of population studies have shown elevated risk for CVD in socially isolated individuals or those with low levels of support, the results are not consistent. A recent meta-analysis found a 50% increase in the odds of survival in persons with stronger social relationships, but there was significant heterogeneity in individual study results.30 Another systematic review revealed a similar pooled effect size for coronary disease (51% increase) related to social isolation and loneliness.1
In cardiac patients, factors such as living alone, lacking a confidant, being socially isolated, or perceiving low support have all been linked to poorer prognosis. In a systematic review of patient populations with coronary disease, such indicators of low social support showed pooled estimates of relative risk ranging from 1.6 to 1.7 for subsequent CVD events and mortality.31
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree