Prophylaxis for Deep Venous Thrombosis
John E. Rectenwald
Thomas W. Wakefield
Despite the serious consequences of deep venous thrombosis (DVT), a recent registry of more than 5,000 patients reported that only 42% of the patients in the study received DVT prophylaxis within 30 days prior to diagnosis of their DVT. In this study, nonsurgical patients were less likely to receive DVT prophylaxis than surgical patients. Clearly, physician awareness of the risks and sequelae of DVT needs to be improved.
Several concepts remain key to proper management and prophylaxis of DVT in patients. First, recognition of underlying risk factors associated with DVT allows for the identification of high-risk patients who would most benefit from prophylaxis. Second, appreciation of the multifactorial nature of DVT may help to identify specific situations in which a patient is at risk for DVT and identify predisposing factors, such as history of DVT or hypercoagulable states. Finally, a good understanding of the natural history of DVT is important in evaluating the risk-to-benefit ratio of anticoagulation and determining the duration of treatment.
Methods of DVT and pulmonary embolism (PE) prophylaxis include pharmacologic, mechanical, and combinations of both methods. Traditionally, prevention of DVT and PE has been accomplished with early postoperative ambulation, pneumatic compression devices (PCD), unfractionated and low-molecular-weight heparins (LMWH), and warfarin sodium. Prevention of PE can also be accomplished by the additional method of vena cava interruption with vena caval filters. The recent development of new therapeutic agents such as fondaparinux (ArixtraTM) offers novel and alternative approaches to anticoagulation therapy that may have a profound impact on the prophylaxis and treatment of DVT and PE in the future.
Pathophysiology
Virchow, in the mid-1800s, postulated that three conditions were of primary importance for venous thrombosis:
Abnormality of venous flow
Abnormality of blood
Vascular injury
These conditions correspond to today’s concepts of stasis, hypercoagulable state, and venous endothelial damage. Although these tenets remain important concepts in the pathogenesis of venous thrombosis, in modern times the origin of DVT is frequently multifactorial and associated with discrete risk factors (Table 66-1). Nonetheless, an adequate understanding of the coagulation cascade and the cellular interactions involved in the genesis of DVT is fundamental to thoughtful evaluation of the patient at risk. A thorough knowledge of the body’s prothrombotic, antithrombotic, and platelet interactions is essential to understanding the mechanisms of action of various therapeutic agents used to prevent and treat DVT.
Table 66-1 Known Risk Factors for DVT and PE | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The molecular interactions involved in venous thrombosis are complicated and require an understanding of the function of the venous endothelium and its interaction with various circulating factors within the blood. Additionally, evidence that thrombosis and inflammation are interrelated is also mounting, and the inflammatory response elicited by venous thrombosis appears to play an important role in the amplification of thrombosis. It is this process that likely leads to the vein wall and valvular damage and to the syndrome of chronic venous insufficiency.
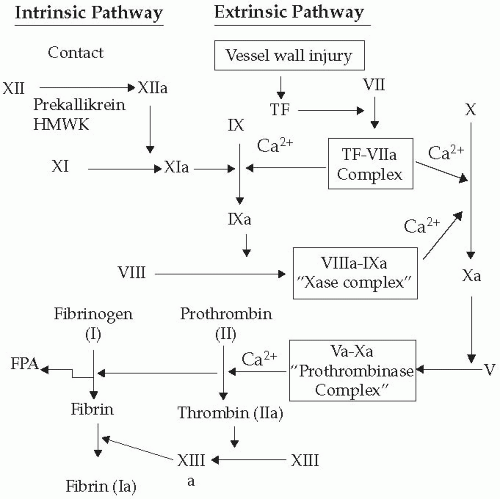
Recently, a four-stage model for development of venous thrombosis has been proposed. Initially, thrombus forms from local procoagulant events, such as small endothelial disruptions at venous confluences or valve pockets. Neutrophils and platelets then activate in the area of injury. In the second stage, further neutrophil and platelet activation occurs on basement membranes that become exposed after endothelial cell disruption. These neutrophils and platelets produce inflammatory and procoagulant mediators that amplify the evolving process. Coagulation complexes such as the Xase and prothrombinase form on the platelet surface, and this greatly accelerates the rate of clot generation in the third stage (Fig. 66-1). Finally, neutrophils, monocytes, and platelets layer on top of the existing thrombus and facilitate clot amplification and the inflammatory response in the fourth stage. This process is very similar to the general process of wound healing. Leukocytes, initially neutrophils followed by monocytes,
associated with these events at the thrombus-vein wall interface, extravasate into the vein wall from both the luminal and adventitial sides. Development of a cytokine/chemokine gradient in the vein wall appears to be responsible for this leukocyte emigration.
associated with these events at the thrombus-vein wall interface, extravasate into the vein wall from both the luminal and adventitial sides. Development of a cytokine/chemokine gradient in the vein wall appears to be responsible for this leukocyte emigration.
Leukocyte-vein wall interactions involve steps including reversible leukocyte rolling and firm adhesion to the endothelium, leukocyte extravasation, and extravascular chemotaxis. Venous injury may occur in response to stasis; venodilation that occurs in procedures such as surgical operations; direct trauma to the vein wall; and importantly, small amounts of thrombin produced through coagulation factor interactions. Neutrophils rapidly interact with both intact endothelium and platelets via the selectin receptors. P and E-selectin are upregulated by thrombin, histamine, tumor necrosis factor, and other cytokines and chemokines, whereas L-selectin is constitutively expressed on neutrophils. This initiates leukocyte cell rolling along the activated venous endothelium, allowing firm adhesion via cellular adhesion molecules (ICAM-1, CD11b/CD18) and subsequent leukocyte extravasation resulting in the venous inflammation associated with DVT. It is assumed that the inflammatory response is detrimental, although the response is also important for thrombus evolution and fibrinolysis. For example, experimentally the presence of neutrophils in the early post-DVT period appears to be critical to limit vein wall fibrosis.
It is not surprising that in a system as complex as coagulation, there are acquired and inherited defects that result in alterations of bleeding, coagulation, and fibrinolysis resulting in an overall procoagulant state favoring DVT formation. Diagnostic tests are available for screening many of these rare imbalances between the coagulation and anticoagulation systems but are expensive. However, these tests may be warranted in patients diagnosed with DVT, as results may be positive in up to 15% of DVT patients less than 45 years of age. Patients with a positive family history of idiopathic thromboembolism, young patients with either arterial or venous thrombosis without known cause, and patients with multiple episodes of thromboembolism without an anatomic abnormality may undergo procoagulant screening with a number of diagnostic tests in an effort to determine the etiology of their DVT.
In summary, vascular hemostasis is a complex process involving an integrated and intricate process of balance between coagulation and anticoagulation pathways, platelet regulation, and complex cellular mechanisms. Clearly the vasculature’s balance between thrombosis and hemorrhage is of great clinical importance to the patient not only on an everyday basis or in the presence of a hemostatic abnormality but especially in times of physiologic stress when the patient is at risk for DVT.
Clinical Considerations
In considering who would benefit from prophylaxis and which type of prophylaxis would be most appropriate, patients may be categorized into levels of risk: low, moderate, high, and highest (Table 66-2). The following information is a summary of the ACCP consensus guidelines of DVT prophylaxis (see “Suggested Readings”). The incidence of calf vein DVT within these categories is expected to be 2%, 10% to 20%, 20% to 40%, and 40% to 80%, respectively, whereas proximal DVT is anticipated to be 0.4%, 2% to 4%, 4% to 8%, and 10% to 20% without prophylaxis. Clinical PE is estimated to occur at rates of 0.2%, 1% to 2%, 2% to 4%, and 4% to 10%, respectively, for these groups and the risk of a fatal PE is appreciably lower at 0.002%, 0.1% to 0.4%, 0.4% to 1%, and 1% to 5%, respectively. General surgical patients are at a 25% risk of DVT overall without prophylaxis. The risk of a clinical PE in these patients is 1.6%, with 0.9% being fatal. These numbers underscore the importance of risk stratification of patients and proper DVT prophylaxis in all groups.
As stated previously, methods of DVT and PE prophylaxis include pharmacologic, mechanical, and combinations of pharmacologic and mechanical therapies. Pharmacologic agents traditionally include standard unfractionated heparin, LMWH, warfarin, dextran, and aspirin. Newer pharmacologic agents such as ximelagatran/melagatran (ExantaTM) and fondaparinux (ArixtraTM) are currently being evaluated and are promising. Mechanical methods include continued or early postoperative ambulation, PCD, and elastic stockings (TED hose). Vena caval interruption with inferior vena cava (IVC) filters offers prophylaxis of PE in patients with contraindications to anticoagulation and known DVT, or when other methods are contraindicated or ineffective.
Risk assessment for DVT can also vary according to what operative procedures a patient undergoes or according to injuries
sustained. It is well known that DVT risk varies for patients; for example, those who are status post total hip arthroplasty or have experienced multisystem trauma are at greater risk of DVT and PE. In fact, the incidence of DVT in patients undergoing orthopedic surgical procedures is as high as 45% to 57% for total hip arthroplasty, 40% to 84% for total knee arthroplasty, and 36% to 60% for hip fracture without prophylaxis. Total PE incidence is cited at 0.7% to 30%, 1.8% to 7%, and 4.3% to 24% for these three groups, respectively, whereas for fatal PE the incidence is 0.34% to 6%, 0.2% to 0.7%, and 3.6% to 12.9% in that order.
sustained. It is well known that DVT risk varies for patients; for example, those who are status post total hip arthroplasty or have experienced multisystem trauma are at greater risk of DVT and PE. In fact, the incidence of DVT in patients undergoing orthopedic surgical procedures is as high as 45% to 57% for total hip arthroplasty, 40% to 84% for total knee arthroplasty, and 36% to 60% for hip fracture without prophylaxis. Total PE incidence is cited at 0.7% to 30%, 1.8% to 7%, and 4.3% to 24% for these three groups, respectively, whereas for fatal PE the incidence is 0.34% to 6%, 0.2% to 0.7%, and 3.6% to 12.9% in that order.
Table 66-2 Factors Associated With Stratification Into Low, Moderate, High, and Highest Risk for DVT | |||
|---|---|---|---|
| |||