Stent design, metal alloy composition, and strut thickness may influence late lumen loss and clinical outcomes after bare metal stent deployment; however, their impact on outcomes after drug-eluting stent deployment is unknown. Although the TAXUS Liberté and ION paclitaxel-eluting stents use similar polymer and drug, the ION stent incorporates a novel thin-strut platinum chromium metal alloy and cell design. We therefore compared patient-level data from 2,298 subjects enrolled into the TAXUS ATLAS (TAXUS Liberté) and PERSEUS (ION) clinical trials. Propensity-score (1:1) matching was performed to adjust for covariate imbalance between stent types. Twelve-month major adverse cardiac events were less frequent after use of the ION compared to the TAXUS Liberté (12.7% vs 8.3%, p <0.001, unadjusted; 12.0% vs 7.5%, p = 0.007, propensity matched) largely because of decreased non–Q-wave myocardial infarction (MI; 2.9% vs 1.4%, p = 0.01, unadjusted; 3.2% vs 0.9%, p = 0.004, propensity matched). The MI difference was predominantly periprocedural and in patients treated with a single stent. In conclusion, this exploratory post hoc analysis demonstrated that the ION was associated with fewer adverse clinical events than the TAXUS Liberté because of decreased non–Q-wave MI. Stent platform-related variables may influence clinical outcomes after drug-eluting stent use despite similar polymer and drug elution. Differences in adjunctive pharmacotherapy and/or stenting technique may also be contributory.
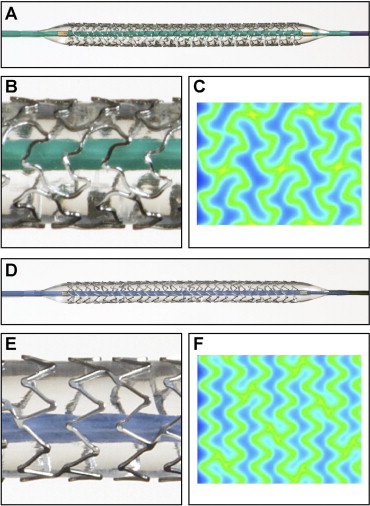
The TAXUS Element (ION) and TAXUS Liberté paclitaxel-eluting stents (PESs) share similar polymer and drug. However, the ION incorporates a novel thin-strut (81 μm) platinum chromium metal alloy and cell design ( Figure 1 ). The Prospective Evaluation in a Randomized Trial of the Safety and Efficacy of the Use of the TAXUS Element Paclitaxel-Eluting Coronary Stent System (PERSEUS) clinical trial program demonstrated noninferiority of the ION compared to the TAXUS Express PES for angiographic and clinical outcomes and superiority of the ION compared to the Express bare metal stent in small vessels. However, no comparison has yet been made between the ION and the more contemporary TAXUS Liberté PES, which incorporates a 316L stainless steel platform with 97 μm strut thickness. We performed a post hoc patient-level propensity-matched exploratory analysis of the pooled Multi-center, Single-arm Study of the TAXUS® Liberté®-SR Stent for the Treatment of Patients with de novo Coronary Artery Lesions (TAXUS ATLAS) and TAXUS PERSEUS clinical trial databases to determine the relative safety and efficacy of the ION and TAXUS Liberté PESs and to investigate the relative influence of stent metal platform variables compared to polymer/drug on outcomes after drug-eluting stent deployment.
Methods
The TAXUS Liberté and ION (Boston Scientific, Natick, Massachusetts) are coronary artery balloon-expandable PESs with SIBS Translute polymer matrix (Boston Scientific, Natick, Massachusetts) containing paclitaxel 1 μg/mm 2 applied to all surfaces of the stent. The ION stent is made of a novel platinum chromium alloy with thin struts (81 μm) designed to decrease recoil and improve flexibility and radiopacity with increased radial strength and increased fracture resistance compared to the TAXUS Liberté stent ( Figure 1 ).
Patient-level data from PES-treated subjects were pooled from the TAXUS ATLAS (TAXUS Liberté stent) and PERSEUS (ION stent) workhorse and small-vessel trials. Individual study designs ( Table 1 ) and 1-year clinical results have been described previously.
| ATLAS Workhorse | ATLAS Small Vessel | PERSEUS Workhorse | PERSEUS Small Vessel | |
|---|---|---|---|---|
| Test device | TAXUS Liberté PES | TAXUS Liberté 2.25-mm PES | ION PES | ION PES |
| Stent material | 316L stainless steel | 316L stainless steel | Platinum chromium alloy | Platinum chromium alloy |
| Device strut thickness (μm) | 97 | 97 | 81–86 ⁎ | 81–86 ⁎ |
| Polymer | Slow-release Translute polymer | Slow-release Translute polymer | Slow-release Translute polymer | Slow-release Translute polymer |
| Study design | Prospective, multicenter, 1 arm, historically controlled | Prospective, multicenter, 1 arm, historically controlled | Prospective, randomized (3:1) controlled | Prospective, multicenter, 1 arm, historically controlled |
| Investigative sites | 61 sites in North America and Asia Pacific | 23 sites in North America and Asia Pacific | 90 sites in United States and Asia Pacific | 28 in United States |
| Enrollment period | August 2004 to February 2005 | February 2005 to March 2006 | July 2007 to October 2008 | July 2007 to August 2008 |
| Randomized to paclitaxel-eluting stent arm | 871 | 261 | 942 | 224 |
| Lesion type | 1 de novo coronary artery | 1 de novo coronary artery | 1 de novo coronary artery | 1 de novo coronary artery |
| Reference vessel diameter inclusion criteria (mm) † | ≥2.5–≤4.0 | ≥2.2–≤2.5 | ≥2.75–≤4.0 | ≥2.25–<2.75 |
| Lesion length inclusion criteria (mm) † | ≥10–≤28 | ≥10–≤28 | ≤28 | ≤20 |
| Number of target lesions and stents allowed | 1 target lesion, single planned study stent | 1 target lesion, single planned study stent | 1 target lesion, single planned study stent | 1 target lesion, single planned study stent |
⁎ Strut thicknesses are 86 μm for the 4.00-mm model and 81 μm for all other models.
All subjects in the PES arms (intent to treat) of these studies were included. Study eligibility criteria have been reported previously and specific inclusion/exclusion criteria are presented in Table 1 . Additional study stents were allowed in the target lesion for bail-out reasons only. Thienopyridine antiplatelet therapy (clopidogrel or ticlopidine) was recommended for a minimum of 6 months in TAXUS ATLAS and 12 months in TAXUS PERSEUS in accordance with guidelines at the time. Aspirin therapy was recommended indefinitely in all studies.
Central analysis of all angiographic studies was performed by an angiographic core laboratory (Beth Israel Deaconess Medical Center, Boston, Massachusetts) using identical standard qualitative morphologic criteria for all studies. An independent clinical events committee adjudicated all reported events of stent thrombosis and potential major adverse cardiac events (MACEs). An independent data monitoring committee provided oversight of aggregate safety data. Protocols were approved by all participating ethics review committees and all patients provided written informed consent. The TAXUS ATLAS workhorse, TAXUS ATLAS small-vessel, TAXUS PERSEUS workhorse, and TAXUS PERSEUS small-vessel studies are registered on the National Institutes of Heath Web site ( http://www.clinicaltrials.gov ) with identifiers NCT00371709 , NCT00371748 , NCT00484315 , and NCT00489541 , respectively.
Target lesion failure was defined as any ischemia-driven target lesion revascularization (TLR), myocardial infarction (MI; Q wave and non-Q wave) related to the target vessel, or cardiac death related to the target vessel. If relation to the target vessel could not be determined with certainty, the event was assumed to be related to the target vessel. MACEs were defined as MI, target vessel revascularization, or cardiac death. MI was defined as Q-wave MI (de novo Q waves in ≥2 leads lasting ≥0.04 second with increased creatine kinase [CK]-MB) or non–Q-wave MI (de novo increase of CK >2.0 times upper limit of normal without new Q waves or positive CK-MB result). Stent thrombosis was defined according to the Academic Research Consortium definite/probable definition.
Continuous variables are expressed as mean ± SD and were compared using t tests. Categorical variables are expressed as number per total (percentage) and were compared by chi-square or Fisher’s exact test. Time-to-event data were analyzed using Kaplan–Meier estimates and groups were compared by log-rank test. Analysis was conducted with SAS ≥9.0 (SAS Institute, Cary, North Carolina).
To adjust baseline differences between the TAXUS Liberté and ION groups, 1:1 propensity-score matching was performed by logistic regression (with stent type as the dependent variable). All patient demographic and baseline lesion variables found to be significantly different between the TAXUS Liberté and ION groups ( Table 2 ) were included. In addition, the following variables were included to balance known risk factors across groups: gender, age, previous coronary artery bypass grafting surgery, diabetes, smoking, hypertension, hyperlipidemia, and postprocedure acute gain. Randomization to the angiographic cohort was included to balance the risk of nonischemia-driven TLR because of the difference in quantitative coronary angiographic follow-up across studies. Patients were matched based on propensity score using the greedy algorithm and all matched patients were included. Unadjusted and propensity-matched data are presented.
| Unadjusted Results | 1:1 Propensity-Matched Results | |||||
|---|---|---|---|---|---|---|
| TAXUS Liberté | ION | p Value | TAXUS Liberté | ION | p Value | |
| (n = 1,132) | (n = 1,166) | (n = 663) | (n = 663) | |||
| Patient demographics and cardiac risk factors | ||||||
| Men | 752/1,132 (66.4%) | 810/1,166 (69.5%) | 0.12 | 464/663 (70.0%) | 462/663 (69.7%) | 0.90 |
| Age (years) | 62.3 ± 10.8 (1,132) | 62.7 ± 9.8 (1,166) | 0.35 | 62.2 ± 10.6 (663) | 62.5 ± 10.0 (663) | 0.65 |
| Previous myocardial infarction | 317/1,132 (28.0%) | 252/1,156 (21.8%) | <0.001 | 166/663 (25.0%) | 169/663 (25.5%) | 0.85 |
| Previous percutaneous coronary intervention | 370/1,132 (32.7%) | 308/1,164 (26.5%) | 0.001 | 187/663 (28.2%) | 191/663 (28.8%) | 0.81 |
| Previous coronary artery bypass grafting | 83/1,132 (7.3%) | 83/1,165 (7.1%) | 0.85 | 44/663 (6.6%) | 41/663 (6.2%) | 0.74 |
| History of heart failure | 51/1,132 (4.5%) | 74/1,159 (6.4%) | 0.048 | 36/663 (5.4%) | 26/663 (3.9%) | 0.19 |
| Unstable angina | 353/1,132 (31.2%) | 240/1,165 (20.6%) | <0.001 | 178/663 (26.8%) | 163/663 (24.6%) | 0.35 |
| Medically treated diabetes | 315/1,132 (27.8%) | 314/1,166 (26.9%) | 0.63 | 169/663 (25.5%) | 172/663 (25.9%) | 0.85 |
| Current smoking | 252/1,132 (22.3%) | 253/1,136 (22.3%) | >0.99 | 155/663 (23.4%) | 155/663 (23.4%) | >0.99 |
| Hyperlipidemia ⁎ | 879/1,132 (77.7%) | 908/1,163 (78.1%) | 0.81 | 505/663 (76.2%) | 503/663 (75.9%) | 0.90 |
| Hypertension † | 826/1,132 (73.0%) | 890/1,165 (76.4%) | 0.06 | 486/663 (73.3%) | 493/663 (74.4%) | 0.66 |
| Baseline lesion characteristics | ||||||
| Baseline vessel diameter (mm) | 2.58 ± 0.56 (1,130) | 2.65 ± 0.53 (1,166) | 0.006 | 2.64 ± 0.54 (663) | 2.65 ± 0.55 (663) | 0.68 |
| Baseline lesion length (mm) | 14.71 ± 6.67 (1,129) | 13.76 ± 6.00 (1,166) | <0.001 | 14.01 ± 6.30 (663) | 14.06 ± 6.11 (663) | 0.90 |
| Baseline minimum lumen diameter (mm) | 0.80 ± 0.34 (1,130) | 0.73 ± 0.33 (1,166) | <0.001 | 0.78 ± 0.343 (663) | 0.78 ± 0.341 (663) | 0.92 |
| Baseline diameter stenosis (%) | 68.70 ± 11.65 (1,130) | 72.37 ± 10.78 (1,166) | <0.001 | 70.30 ± 11.33 (663) | 70.57 ± 10.81 (663) | 0.65 |
| Bend >45° | 317/1,132 (28.0%) | 139/1,166 (11.9%) | <0.001 | 108/663 (16.3%) | 120/663 (18.1%) | 0.38 |
| Type B2/C lesions | 837/1,132 (73.9%) | 760/1,166 (65.2%) | <0.001 | 458/663 (69.1%) | 467/663 (70.4%) | 0.59 |
| Calcification | 331/1,129 (29.3%) | 258/1,166 (22.1%) | <0.001 | 155/663 (23.4%) | 171/663 (25.8%) | 0.31 |
| Procedural characteristics | ||||||
| In-stent acute gain (mm) | 1.63 ± 0.49 (1,120) | 1.85 ± 0.43 (1,162) | <0.001 | 1.75 ± 0.45 (663) | 1.76 ± 0.43 (663) | 0.70 |
| Average study stents (number) | 1.07 ± 0.31 (1,132) | 1.07 ± 0.31 (1,166) | 0.85 | 1.05 ± 0.24 (663) | 1.07 ± 0.32 (663) | 0.21 |
| Total stented length (mm) | 22.22 ± 8.31 (1,125) | 19.95 ± 7.93 (1,163) | <0.001 | 21.24 ± 7.03 (663) | 20.34 ± 8.30 (663) | 0.03 |
| Multiple stents | 85/1,132 (7.5%) | 90/1,166 (7.7%) | 0.85 | 39/663 (5.9%) | 55/663 (8.3%) | 0.09 |
| Maximum pressure overall (atm) | 15.57 ± 3.29 (1,131) | 15.88 ± 3.01 (1,164) | 0.02 | 15.54 ± 3.34 (662) | 16.00 ± 3.01 (662) | 0.008 |
| Maximum postdilatation pressure (atm) | 16.39 ± 4.02 (520) | 16.50 ± 3.60 (631) | 0.61 | 16.50 ± 3.84 (303) | 16.65 ± 3.60 (377) | 0.58 |
⁎ History of hyperlipidemia requiring medication.
Results
In total 2,298 PES-treated subjects were included (1,132 in TAXUS Liberté arm and 1,166 in ION arm). After 1:1 propensity matching, there were 663 patients per group.
Unadjusted and propensity-matched baseline demographic and lesion characteristics are listed in Table 2 . Several significant differences between the ION and TAXUS Liberté groups were consistent with increased baseline risk in the TAXUS Liberté arm and included increased rates of previous MI and percutaneous coronary intervention, a higher incidence of unstable angina, smaller baseline reference vessel diameter, longer lesion length, more lesions in a vessel with a bend >45°, American Heart Association/American College of Cardiology type B2/C lesions, and calcification. In contrast, baseline minimum lumen diameter was significantly smaller and baseline percent diameter stenosis significantly greater in the ION arm. After 1:1 propensity matching, there were no significant differences in patient demographics or lesion characteristics between stent types.
Of note, more frequent protocol-mandated angiographic follow-up occurred in the TAXUS Liberté arm (71.0% vs 41.2%, p <0.001). The portion of patients with angiographic follow-up was balanced between stent types after propensity matching (58.7% vs 57.5%, p = 0.66).
Aspirin and thienopyridine use and statin therapy were similar between treatment arms at hospital discharge in the propensity-matched cohort ( Table 3 ). At 1 year after stenting, although aspirin use was similar, unadjusted rates of clopidogrel or ticlopidine use were significantly greater after ION versus TAXUS Liberté stenting ( Table 3 ) because of revised treatment guidelines over time.
| Unadjusted Results | 1:1 Propensity-Matched Results | |||||
|---|---|---|---|---|---|---|
| TAXUS Liberté | ION | p Value | TAXUS Liberté | ION | p Value | |
| (n = 1,132) | (n = 1,166) | (n = 663) | (n = 663) | |||
| Periprocedural | ||||||
| Aspirin | 1,121/1,132 (99.0%) | 1,110/1,166 (95.2%) | <0.001 | 654/663 (98.6%) | 624/663 (94.1%) | <0.001 |
| Clopidogrel or ticlopidine | 1,131/1,132 (99.9%) | 1,158/1,166 (99.3%) | 0.04 | 662/663 (99.8%) | 660/663 (99.5%) | 0.62 |
| Heparin | 1,130/1,132 (99.8%) | 1,159/1,166 (99.4%) | 0.18 | 663/663 (100.0%) | 660/663 (99.5%) | 0.25 |
| Glycoprotein IIb/IIIa inhibitor | 263/1,132 (23.2%) | 158/1,166 (13.6%) | <0.001 | 168/663 (25.3%) | 90/663 (13.6%) | <0.001 |
| After procedure through discharge | ||||||
| Aspirin | 1,128/1,131 (99.7%) | 1,163/1,164 (99.9%) | 0.37 | 663/663 (100.0%) | 661/662 (99.8%) | 0.50 |
| Clopidogrel or ticlopidine | 1,128/1,129 (99.9%) | 1,162/1,165 (99.7%) | 0.62 | 661/662 (99.8%) | 662/663 (99.8%) | >0.99 |
| Angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker | 604/1,130 (53.5%) | 731/1,165 (62.7%) | <0.001 | 346/663 (52.2%) | 412/663 (62.1%) | <0.001 |
| β Blocker | 788/1,131 (69.7%) | 836/1,165 (71.8%) | 0.27 | 448/663 (67.6%) | 479/663 (72.2%) | 0.06 |
| Warfarin | 27/1,131 (2.4%) | 41/1,165 (3.5%) | 0.11 | 15/663 (2.3%) | 22/663 (3.3%) | 0.24 |
| Statins | 983/1,131 (86.9%) | 1,048/1,165 (90.0%) | 0.02 | 579/663 (87.3%) | 598/663 (90.2%) | 0.10 |
| Discharge | ||||||
| Aspirin | 1,128/1,131 (99.7%) | 1,162/1,163 (99.9%) | 0.37 | 663/663 (100.0%) | 661/662 (99.9%) | 0.50 |
| Clopidogrel or ticlopidine | 1,128/1,129 (99.9%) | 1,162/1,164 (99.8%) | >0.99 | 661/662 (99.9%) | 662/663 (99.9%) | >0.99 |
| At 12 months | ||||||
| Aspirin | 1,053/1,097 (96.0%) | 1,088/1,128 (96.5%) | 0.57 | 618/645 (95.8%) | 618/641 (96.4%) | 0.58 |
| Clopidogrel or ticlopidine | 613/1,097 (55.9%) | 1,038/1,128 (92.0%) | <0.001 | 354/645 (54.9%) | 594/641 (92.7%) | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


