Serum uric acid (UA) is associated with death and hospitalization in chronic heart failure (HF). However, UA in acute HF has not been well studied with respect to its relation to renal dysfunction and vascular congestion. We measured admission serum UA along with baseline variables in 281 patients with acute HF screened from the Loop Diuretics Administration and Acute Heart Failure (Diur-HF) trial. Hyperuricemia was defined as serum UA >7 mg/dl in men and >6 mg/dl in women. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate <60 ml/min/1.73 m 2 before hospital admission. Death or HF hospitalization at 6 months was the primary outcome. The mean UA concentration was 6.4 ± 2.5 mg/dl, and 121 patients (43.1%) were classified as hyperuricemic. UA values were significantly increased in patients with CKD compared to patients without CKD (6.8 ± 2.7 vs 6.1 ± 2.1 mg/dl; p = 0.02); however, UA was not associated with the development of acute kidney injury. Patients with hyperuricemia had greater degrees of pulmonary and systemic congestion than normouricemic patients (congestion score 3.5 vs 2.1, p <0.01). Hyperuricemia was associated with higher risk of death or HF rehospitalization (univariate hazard ratio 1.46 [1.02 to 2.10]; p = 0.04, multivariate hazard ratio 1.69 [1.16 to 2.45]; p = 0.005). In conclusion, hospitalized patients with acute HF, elevated UA levels were associated with both CKD and pulmonary congestion. After controlling for potential confounders, hyperuricemia was associated with rehospitalization and death at 6 months.
Uric acid (UA) is the final product of purine catabolism and is excreted by the kidneys. The enzymes responsible of UA metabolism and production are xanthine oxidase (XO) and xanthine dehydrogenase. Both enzymes catalyze the oxidation hypoxanthine to xanthine; however, most of purines are metabolized by XO particularly during hypoxia, acidosis, and inflammation. During this process, reactive oxygen species and the hydroxyl radical are generated. Free radicals produced lead to reduced nitric oxide release and synthesis with increased oxidative stress. In addition, oxidative stress and nitric oxide imbalance could enhance inflammatory pathways and further increase cytokine production. Although data regarding XO expression in the myocardium and vasculature are controversial, many nonhuman studies suggest that XO polymorphisms have physiological effects on the vascular endothelium. In patients with heart failure (HF) and associated hyperuricemia, both oxidative stress and proinflammatory cytokines could influence clinical symptoms by reducing the anaerobic threshold, depleting adenosine triphosphate in the sarcoplasmic reticulum, and impairing both systolic and diastolic energetics. Accordingly, many reports have demonstrated the relation between increased UA levels and functional class, reduced exercise tolerance, systemic congestion, and decreased left ventricular function in patients with chronic HF. Little is known, however, about the association if any between elevated UA concentration and outcomes in acute HF after controlling for chronic kidney disease (CKD), acute kidney injury (AKI), pulmonary congestion, and relative intravascular volume expansion. Thus, in this study, we sought to explore the relations between baseline UA, CKD, and congestion and to determine the independent relations, if any, between these variables and the outcome of HF hospitalization or death at 6 months.
Methods
This was a retrospective single-center study including consecutively 281 subjects, screened from the Loop Diuretics Administration and Acute Heart Failure (Diur-HF) trial ( NCT01441245 ) from January 2011 to October 2014. Patients had a primary diagnosis of acute HF, left ventricular ejection fraction <45%, with evidence of volume overload and were within <24 hours from hospital presentation defined as pulmonary/systemic congestion as assessed by 2 independent clinicians. A congestion score was evaluated considering 5 principal signs and giving for each sign 1 point in a scale ranging from 1 to 5: the clinical signs considered were: third heart sound, pulmonary rales, jugular venous stasis, hepatomegaly, and peripheral edema. Exclusion criteria were end-stage renal disease or the need for a renal replacement therapy (dialysis or ultrafiltration), recent myocardial infarction, systolic blood pressure <90 mm Hg, serum creatinine level >4.0 mg/dl, sepsis, systemic inflammatory diseases, severe liver, disease, or neoplastic disease. Results from the Diur-HF Trial were published elsewhere but in brief, 92 (from 281 screened) subjects were randomized to a continuous infusion versus bolus strategy for loop diuretic administration. The overall findings were mixed with continuous infusion associated with greater reductions in B-type natriuretic peptide (BNP). However, this appeared to occur at the consequence of worsened renal filtration function, the use of additional treatment, and higher rates of rehospitalization or death at 6 months. This study included the 281 screened with baseline laboratories available and was approved by our local institutional review board, and all patients gave their signed informed consent. We defined as hyperuricemia as serum UA >7 mg/dl in men and >6 mg/dl in women. CKD was defined as estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m 2 at baseline. The eGFR was calculated using the Modification of Diet in Renal Disease equation. AKI was defined as creatinine increase ≥0.3 mg/dl at any time from admission to discharge or eGFR decrease ≥20% from baseline at any time.
Serum creatinine (Jaffe reaction; CREJ2 cobas; Roche system, Mannheim, Germany), UA, blood urea nitrogen, and BNP were assessed with 24 hours from admission and at discharge at a central laboratory. UA measurement was performed by enzymatic colorimetric test where uricase cleaves UA to form allantoin and hydrogen peroxide. In the presence of peroxidase, 4-aminophenazone is oxidized by hydrogen peroxide to a quinone-diamine dye. The color intensity of the quinone-diamine formed is directly proportional to the UA concentration and is determined by measuring the increase in absorbance (UA 2; Cobas; Roche system). Plasma BNP was measured with Triage BNP test (Biosite Inc., San Diego, California).
The primary outcome was death or hospitalization at 6 months. A secondary outcome was the occurrence of AKI during hospitalization. Patients were followed for 6 months after discharge with clinical visits or telephone contacts. If the hospitalization was not for HF, but related events such as pump failure, acute coronary syndromes complicated by HF, ventricular arrhythmias associated with left ventricular dysfunction, or HF associated with worsened renal function, for analytical purposes these were all considered as hospitalization for HF.
Continuous variables are expressed as mean (±SD), whereas discrete variables are presented as counts with proportions. Patients’ laboratory parameters were compared using the Student t test for independent groups. Analysis of variance was done by the Levine’s test. For discrete variables, we used the chi-square test. Receiver operating characteristic curve analysis was used to assess the prognostic value of UA through a range of values on the primary outcome. Cox regression analysis was used to assess the independent effect of baseline UA on rehospitalization and death at 6 months. The treatment assignment in the Diur-HF trial was not included as a covariate because our study included more than half of patients who were screened and not randomized. Kaplan–Meier methods were used to generate survival plots that compared the 2 groups using the log-rank test for the time to first occurrence of HF hospitalization or death. All reported probability values were 2-tailed, and a p value ≤0.05 was considered statistically significant. Statistical analysis was performed using the SPSS 20.0 statistical software package (SPSS Inc., Chicago, Illinois). Using the rates of primary events that had occurred by 6 months, our study had an observed power of 52% to observe a relative risk of 1.25 with an assumed alpha error of 0.05 and a sample size of 140 subjects in each group.
Results
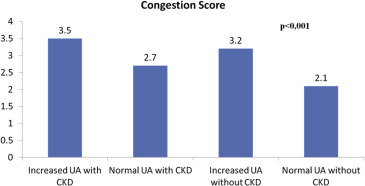
A total of 281 patients with acute HF were evaluated, and we lost 11 because of sudden death, 8 for incomplete data during follow-up. A total of 121 of 281 patients (43.1%) met the definition of hyperuricemia. The resulting study sample is described in Table 1 . Mean congestion scores stratified by UA levels and CKD are shown in Figure 1 .
| Parameters | Uric Acid Level Elevated (N=121) | Normal (N=141) | p-value |
|---|---|---|---|
| Age (years) | 81±6 | 81±4 | 0.851 |
| Men | 49 (41%) | 65 (%) | 0.431 |
| Creatinine (mg/dl) | 1.6±0.5 | 1.3±0.4 | 0.05 |
| Blood urea nitrogen (mg/dl) | 81±50 | 72±46 | 0.141 |
| Estimated glomerular filtration rate ( ml/min/1.73 m 2 ) | 46±13 | 55±10 | 0.02 |
| B-type natriuretic peptide (pg/ml) | 948±929 | 899±868 | 0.733 |
| Albumin (g/dL) | 3.5±0.5 | 3.1±0.5 | 0.002 |
| Hemoglobin (g/dL) | 11.9±1.8 | 11.3±1.9 | 0.02 |
| Uric acid (mg/dL) | 8.5±1.7 | 4.7±1.1 | <0.001 |
| Ejection fraction (%) | 38±9 | 40±9 | 0.486 |
| Congestion signs | 3.5±1.0 | 2.2±1.0 | <0.001 |
| Admission blood urea nitrogen >50 mg/dL | 94 (78%) | 92 (65%) | 0.02 |
| Chronic kidney disease | 67 (55%) | 59 (42%) | 0.04 |
| Current smoking | 30 (25%) | 42 (30%) | 0.374 |
| Dyslipidemia | 56 (46%) | 63 (45%) | 0.991 |
| Diabetes mellitus | 44 (36%) | 34 (24%) | 0.04 |
| Hypertension | 69 (57%) | 39 (28%) | <0.001 |
| Atrial fibrillation | 56 (46%) | 38 (30%) | 0.001 |
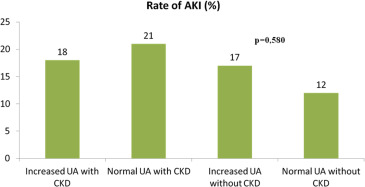
The rates of AKI were 20 (17%) versus 22 (16%), for those with and without hyperuricemia, respectively, p = 0.904. AKI rates stratified UA levels, and CKD are reported in Figure 2 . The rates of HF hospitalization, death, and the composite end point were 61 (50%) versus 41 (29%), p <0.001; 36 (30%) versus 27 (19%) p = 0.045; and 97 (80%) versus 68 (48%) p <0.001; for those with and without hyperuricemia, respectively. Among those with CKD, the rates for the composite outcome were 72 (58%) and 69 (50%), for those with and without hyperuricemia, respectively, p = 0.155. Among those without CKD, the rates for the composite outcome were 45 (68%) and 28 (51%), for those with and without hyperuricemia, respectively, p = 0.05. Using survival analysis, hyperuricemia was a significant univariate predictor of the primary end point (hazard ratio [HR] 1.46 [1.02 to 2.10]; p = 0.04). Using the Cox proportional hazards model and adjusting for age, gender, the presence of CKD, blood urea nitrogen, BNP, congestion, anemia, hypertension, dyslipidemia, smoking, diabetes, peripheral atherosclerosis, and coronary artery disease, we found that hyperuricemia remained significantly associated with the primary end point (HR 1.69 [1.16 to 2.45]; p = 0.005; Table 2 ). The Kaplan–Meier survival curves for those with and without hyperuricemia are shown in Figure 3 , (log-rank p = 0.009). Dividing into 3 UA level groups (<6 mg/dl, 6 8 mg/dl, >8 mg/dl), we found that there was a significant relation between survival and UA levels <6 mg/dl but no graded affect above that concentration (log-rank p = 0.03) as shown in Figure 4 . Receiver operating characteristic curve analysis demonstrated that baseline UA concentrations were significant predictors of HF hospitalization or death (area under the curve 0.70 [0.64 to 0.77], p <0.001, Figure 5 ).
| Parameters | Univariate | Multivariate ∗ | ||
|---|---|---|---|---|
| HR (95% CI of HR) | p-value | HR (95% CI of HR) ∗ | p-value | |
| Hyperuricemia | 1.46 (1.02-2.10) | 0.04 | 1.69 (1.16-2.45) | 0.005 |
| Acute kidney injury | 0.73 (0.44-1.22) | 0.23 | 0.76 (0.45-1.24) | 0.27 |
| Blood urea nitrogen > 50 mg/dL | 1.46 (0.94-2.25) | 0.09 | 1.46 (0.94-2.26) | 0.08 |
| B-type natriuretic peptide > 400 pg/mL | 0.88 (0.57-1.38) | 0.59 | 0.81 (0.52-1.28) | 0.37 |
| Left ventricular ejection fraction < 40% | 0.98 (0.61-1.56) | 0.93 | 1.02 (0.64-1.61) | 0.93 |
| Signs of vascular congestion at admission | 1.11 (0.89-1.38) | 0.36 | 1.09 (0.87-1.38) | 0.42 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


