Elevated systolic pulmonary artery pressure (SPAP) after ST-segment elevation myocardial infarction (STEMI) has been associated with adverse outcome. However, little is known about the development of increased SPAP after STEMI treated with primary percutaneous coronary intervention. The aims of this study were to investigate the incidence and determinants of elevated SPAP (SPAP ≥36 mm Hg at 12 months) after first STEMI and to analyze its prognostic implications. A total of 705 patients (60 ± 12 years; 75% men; left ventricular ejection fraction [LVEF] 47 ± 9%) with first STEMI treated with primary percutaneous coronary intervention were evaluated. Two-dimensional echocardiography was available at baseline and 12-month follow-up. Data on all-cause mortality were collected at long-term follow-up. Incident elevated SPAP was present in 5% (n = 38) of patients. Patients with incident elevated SPAP were older (66 ± 12 vs 60 ± 11 years, p = 0.001), had more systemic hypertension (58% vs 30%, p <0.001) and lower LVEF (43 ± 9% vs 48 ± 8%, p <0.001) than their counterparts. Left atrial volume was larger (23 ± 11 vs 18 ± 6 ml/m 2 , p = 0.006), and moderate to severe mitral regurgitation was more prevalent in patients with incident elevated SPAP (16% vs 7%, p = 0.05). Independent correlates of incident elevated SPAP at 12-month follow-up were age (odds ratio [OR] 1.04, 95% CI 1.01 to 1.08, p = 0.01), hypertension (OR 2.52, 95% CI 1.23 to 5.14, p = 0.01), baseline LVEF (OR 0.94, 95% CI 0.90 to 0.98, p = 0.003), and baseline left atrial volume (OR 1.08, 95% CI 1.03 to 1.12, p = 0.001). Incident elevated SPAP was independently associated with all-cause mortality (hazard ratio 3.84, 95% CI 1.76 to 8.39, p = 0.001). In conclusion, although the incidence of elevated SPAP after STEMI is low, its presence is independently associated with increased risk of all-cause mortality at follow-up.
The presence of elevated systolic pulmonary artery pressure (SPAP) after ST-segment elevation myocardial infarction (STEMI) has been associated with adverse outcome. In addition, elevated SPAP may develop after STEMI due to increased left ventricular (LV) filling pressures due to LV systolic and diastolic dysfunction. The adoption of primary percutaneous coronary intervention (PCI) as standard treatment of STEMI has led to limited infarct size, more preserved LV systolic and diastolic function and less frequently adverse LV remodeling at follow-up, which may result in lower incidence of increased SPAP. However, this hypothesis remains unexplored. Furthermore, little is known about the development and the prognostic implications of incident elevated SPAP during follow-up after STEMI. Accordingly, the aims of the present evaluation were threefold (1) to assess the prevalence of elevated SPAP at baseline and at 12-month follow-up in patients with STEMI treated with primary PCI; (2) to assess contributing factors to the development of incident elevated SPAP during follow-up; and (3) to evaluate the prognostic implications of incident elevated SPAP during follow-up after STEMI.
Methods
The present evaluation included 705 patients with a first STEMI treated with primary PCI and with available echocardiograms at baseline and 12-month follow-up, in which measurement of SPAP was feasible. Patients with previous MI were excluded. Other exclusion criteria were out-of-hospital cardiac arrest, severe chronic obstructive pulmonary disease, lobectomy, preexisting pulmonary arterial hypertension, congenital heart disease, and preexisting severe valvular heart disease. Finally, patients with reinfarction before the 12-month echocardiogram were excluded. For this retrospective analysis of clinically acquired data, the institutional review board waived the need of patient written informed consent.
Images were obtained with the patient at rest in the left lateral decubitus position using a commercially available system (Vivid 7 and E9; General Electric-Vingmed, Horten, Norway). Data were acquired with 3.5 MHz or M5S transducers in the standard parasternal and apical views. Standard M-mode, 2-dimensional, color, pulsed, and continuous wave Doppler images were obtained and stored in cineloop format. Data analysis was performed offline with commercially available postprocessing data software (EchoPAC version 111.0.0; General Electric-Vingmed). SPAP was calculated by adding right ventricular (RV) pressure to right atrial pressure. RV pressure was estimated by calculating the systolic pressure gradient between the RV and right atrium by the maximum velocity of the regurgitant jet using the modified Bernoulli equation. Right atrial pressure was estimated by measuring the diameter and the inspiratory collapse of the vena cava inferior. Furthermore, RV dimensions and function were evaluated by measuring RV end-diastolic area, RV end-systolic area, right atrial area, and tricuspid annular plane systolic excursion in the apical 4-chamber view. In addition, LV volumes and function were assessed by measuring the LV end-systolic and end-diastolic volumes, indexed to the body surface area, and LV ejection fraction (LVEF) using the biplane Simpson’s method. The wall motion score index was calculated as recommended by current guidelines. LV diastolic function was evaluated by obtaining peak early (E) and late (A) diastolic velocities and E-wave deceleration time. E′ was assessed with tissue Doppler imaging at the septal side of the mitral annulus in the apical 4-chamber view, and E/E′ ratio was calculated. Moreover, according to the biplane Simpson’s technique maximal left atrial volume was measured in the apical 4-chamber and 2-chamber views and indexed to the body surface area. The presence of significant valvular heart disease was evaluated according to the current guidelines.
After discharge, patients were followed up according to the institutional MISSION! protocol. The study end point was all-cause mortality with the initial time set at the 12-month echocardiogram. Survival status was obtained through municipal civil registries. Lost to follow-up was considered in patients without data on the last 6 months. Data of these patients were included up to the last date of follow-up.
Continuous data are reported as mean ± SD or SEM, where appropriate, and categorical data as frequencies and percentages. Elevated SPAP was defined as ≥36 mm Hg. The prevalence of elevated SPAP was assessed at baseline and at 12-month follow-up. Incident elevated SPAP at 12-month follow-up was defined as normal SPAP at baseline and elevated SPAP at the 12-month echocardiogram. Thereafter, patients with elevated SPAP at both baseline and 12-month follow-up were excluded from further analyses. Subsequently, the patient population was divided according to the presence or absence of incident elevated SPAP at 12-month follow-up. Differences in baseline clinical and echocardiographic characteristics between patients with and without incident elevated SPAP were assessed using the Student t test and chi-square test. Univariate and multivariate logistic regression analyses were performed to identify independent baseline clinical and echocardiographic correlates of incident elevated SPAP. Thereafter, cumulative survival rates were assessed using the Kaplan–Meier method. The initial time was set at the 12-month echocardiogram, and the end of follow-up was set at the date of death from any cause or censored at December 31, 2012. Using a log-rank test, differences in cumulative survival rates in patients with or without incident elevated SPAP were assessed. Finally, univariate and multivariate Cox proportional hazards analyses were performed to identify clinical and 12-month echocardiographic determinants of all-cause mortality, and the relative risks were expressed as hazard ratios with associated 95% confidence intervals. Clinical and echocardiographic relevant univariate variables with a p <0.10 were included in a multivariate Cox regression model. For multivariate models, all univariate variables with a p <0.10 were included in a multivariate logistic regression model. A correlation coefficient of <0.7 was set to avoid multicollinearity between univariate correlates. To avoid multicollinearity between univariate parameters, a correlation coefficient of <0.7 was set. In addition, LV end-diastolic volume, LV end-systolic volume, and LVEF were not included in the same model to avoid collinearity. Likewise, different parameters of diastolic function were not included in the same model. A p value <0.05 was considered statistically significant, and all tests were 2 sided. All statistical analyses were performed with SPSS software, version 20.0 (SPSS Inc., Chicago, Illinois).
Results
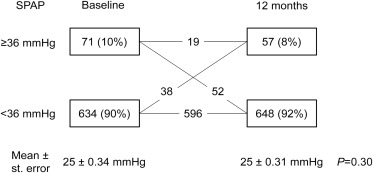
A total of 705 patients with a first STEMI treated with PCI were evaluated. Table 1 summarizes the baseline clinical and echocardiographic characteristics of the patient population. In the overall population, there were no significant changes in SPAP at baseline and 12-month follow-up (25 ± 9 mm Hg and 25 ± 8 mm Hg, respectively). The prevalence of elevated SPAP (defined as ≥36 mm Hg) at baseline and 12-month follow-up was 10% (n = 71) and 8% (n = 57), respectively ( Figure 1 ). Nineteen patients with elevated SPAP at baseline and 12-month follow-up were hereafter excluded from further analyses ( Figure 1 ). The incidence of elevated SPAP at follow-up was 5% (n = 38) and were hereafter referred to as patients with incident elevated SPAP.
| Variable | Overall population (n = 705) | Systolic pulmonary arterial pressure at follow-up | P ∗ | |
|---|---|---|---|---|
| Normal (n = 648 † ) | Elevated (n = 38 † ) | |||
| Age (years) | 60 ± 12 | 60 ± 11 | 66 ± 12 | 0.001 |
| Men | 529 (75%) | 485 (75%) | 29 (76%) | 0.84 |
| Current smoker | 353 (50%) | 325 (50%) | 17 (45%) | 0.51 |
| Diabetes mellitus | 59 (8%) | 56 (9%) | 2 (5%) | 0.76 |
| Family history of coronary artery disease | 320 (45%) | 294 (45%) | 20 (53%) | 0.41 |
| Hypercholesterolemia | 126 (18%) | 116 (18%) | 8 (21%) | 0.63 |
| Hypertension | 222 (32%) | 193 (30%) | 22 (58%) | <0.001 |
| Culprit coronary artery | ||||
| Left anterior descending | 329 (47%) | 298 (46%) | 15 (39%) | 0.52 |
| Right | 270 (38%) | 251 (39%) | 17 (45%) | 0.38 |
| Left circumflex | 105 (15%) | 99 (15%) | 5 (13%) | 0.77 |
| Multivessel coronary disease | 335 (48%) | 304 (47%) | 19 (50%) | 0.71 |
| Thrombolysis In Myocardial Infarction 2 – 3 flow | 698 (99%) | 642 (99%) | 38 (100%) | 1.00 |
| Killip class ≥2 | 20 (3%) | 18 (3%) | 2 (5%) | 0.31 |
| Peak creatine phosphokinase level (U/L) | 2,107 ± 1,959 | 2,042 ± 1,906 | 2,304 ± 2,201 | 0.42 |
| Peak troponin T level (μg/L) | 5.6 ± 5.4 | 5.3 ± 5.1 | 7.0 ± 7.5 | 0.06 |
| Medication at discharge | ||||
| Angiotensin-converting enzyme -inhibitors / angiotensin receptor blockers | 691 (98%) | 636 (98%) | 37 (97%) | 0.53 |
| Antiplatelets | 705 (100%) | 648 (100%) | 38 (100%) | 1.00 |
| Beta-blockers | 666 (95%) | 613 (95%) | 35 (92%) | 0.51 |
| Statins | 701 (99%) | 644 (99%) | 38 (100%) | 1.00 |
| Echocardiography | ||||
| Left ventricular end-systolic volume (mL/m 2 ) | 28 ± 9 | 28 ± 9 | 31 ± 13 | 0.14 |
| Left ventricular end-diastolic volume (mL/m 2 ) | 52 ± 14 | 52 ± 14 | 53 ± 17 | 0.81 |
| Left ventricular ejection fraction (%) | 47 ± 9 | 48 ± 8 | 43 ± 9 | <0.001 |
| Wall motion score index | 1.44 ± 0.28 | 1.43 ± 0.27 | 1.49 ± 0.27 | 0.24 |
| Mitral inflow peak early velocity/mitral inflow peak late velocity ratio | 0.99 ± 0.37 | 0.97 ± 0.36 | 1.03 ± 0.46 | 0.33 |
| Deceleration time (ms) | 207 ± 71 | 209 ± 71 | 192 ± 73 | 0.17 |
| Mitral inflow peak early velocity/mitral annular peak early velocity ratio | 13 ± 5 | 13 ± 5 | 14 ± 4 | 0.12 |
| Left atrial volume (mL/m 2 ) | 18 ± 7 | 18 ± 6 | 23 ± 11 | 0.006 |
| Moderate to severe mitral regurgitation grade, n (%) | 51 (7%) | 43 (7%) | 6 (16%) | 0.05 |
| Tricuspid annulus diameter (cm) | 3.7 ± 0.6 | 3.7 ± 0.6 | 3.8 ± 0.6 | 0.17 |
| Right ventricular end-diastolic area (cm 2 ) | 18.1 ± 4.3 | 18.2 ± 4.3 | 18.4 ± 4.5 | 0.80 |
| Right ventricular end-systolic area (cm 2 ) | 10.4 ± 2.9 | 10.4 ± 2.9 | 10.6 ± 3.2 | 0.63 |
| Tricuspid annular plane systolic excursion (mm) | 19 ± 3.3 | 19 ± 3.3 | 20 ± 3.6 | 0.06 |
| Right atrial area (cm 2 ) | 13.1 ± 3.9 | 13.0 ± 3.7 | 13.6 ± 5.1 | 0.48 |
| Moderate to severe tricuspid regurgitation grade, n (%) | 41 (6%) | 31 (5%) | 4 (11%) | 0.12 |
| Estimated systolic pulmonary arterial pressure (mm Hg) | 24.9 ± 8.9 | 24.2 ± 8.3 | 26.7 ± 6.8 | 0.07 |
∗ p Values are provided for the comparisons of patients with and without incident elevated systolic pulmonary arterial pressure (SPAP) at 12-month follow-up (defined as SPAP ≥36 mm Hg).
† For this comparison, patients with elevated SPAP at both baseline and 12-month follow-up were excluded (n = 19).
Baseline characteristics of patients with and without incident elevated SPAP are presented in Table 1 . Patients with incident elevated SPAP were significantly older and had a higher prevalence of hypertension in comparison to patients with normal SPAP. Both groups were comparable for other cardiovascular risk factors, culprit vessel and mean peak cardiac enzymes although patients with incident elevated SPAP tended to show higher peak cardiac troponin T levels. At discharge, there was no difference in use of medication ( Table 1 ).
Regarding echocardiographic characteristics, patients with incident elevated SPAP showed a lower LVEF and larger left atrial volumes at baseline compared with their counterparts ( Table 1 ). Furthermore, in patients with incident elevated SPAP, there was a higher prevalence of moderate to severe mitral regurgitation grade compared with the group with normal SPAP. There were no significant differences in RV volumes and function at baseline between the 2 groups ( Table 1 ).
Table 2 demonstrates the univariate and multivariate logistic regression analyses to identify correlates of incident elevated SPAP at 12-month follow-up. Age, hypertension, peak cardiac troponin T level, LVEF, and left atrial volume (all with p <0.10 at univariate analysis) were included in the final multivariate model. Independent determinants of incident elevated SPAP were age, hypertension, LVEF, and left atrial volume ( Table 2 ).
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% Confidence interval | P | Odds ratio | 95% Confidence interval | P | |
| Age (years) | 1.05 | 1.02 – 1.09 | 0.001 | 1.04 | 1.01 – 1.08 | 0.01 |
| Hypertension | 3.23 | 1.66 – 6.29 | 0.001 | 2.52 | 1.23 – 5.14 | 0.01 |
| Peak troponin T level (μg/L) | 1.05 | 0.99 – 1.10 | 0.07 | 1.02 | 0.96 – 1.08 | 0.58 |
| Left ventricular end-systolic volume (mL/m 2 ) | 1.03 | 1.00 – 1.06 | 0.05 | |||
| Left ventricular ejection fraction (%) | 0.93 | 0.90 – 0.97 | 0.001 | 0.94 | 0.90 – 0.98 | 0.003 |
| Moderate to severe mitral regurgitation grade | 2.64 | 1.05 – 6.65 | 0.04 | |||
| Left atrial volume (mL/m 2 ) | 1.10 | 1.05 – 1.14 | <0.001 | 1.08 | 1.03 – 1.12 | 0.001 |
| Tricuspid annular plane systolic excursion (mm) | 1.10 | 0.99 – 1.22 | 0.06 | |||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


