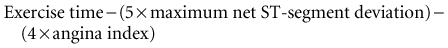4 Prior Evaluation
Functional Testing, Multidetector CT
 Stress Testing
Stress Testing
Heart rate recovery is another index that appears to be related to autonomic tone. Most evidence suggests that a rapid reactivation of vagal tone is the major determinant of a decline in heart rate during the first 30 seconds to 1 minute after exercise. Unlike chronotropic incompetence, heart rate recovery is not significantly affected by the administration of beta blockers. Heart rate recovery is calculated as the difference in heart rate at peak versus 1 minute after exercise. A value <12 beats per minute is considered abnormal. Patients evaluated for suspected or known CAD with an abnormal heart rate recovery have a markedly increased mortality rate that is independent of other risk factors.1 Although both impaired chronotropic response and heart rate recovery are powerful predictors of outcomes, it is unknown whether these are modifiable. Moreover, their association with mortality may be independent of the presence or severity of CAD. Therefore they may have limited value in guiding therapeutic interventions.
ECG Stress Testing
Maximum net ST-segment deviation is defined as the maximum deviation (elevation or depression) noted in any of the 12 ECG leads as compared with baseline. The treadmill anginal index is defined as having a value of 0 if no angina occurs, 1 if angina occurs during exercise but is not test-limiting, or 2 if test-limiting angina occurs. Exercise time is measured based on the Bruce protocol and appears to be the most important determinant of prognosis.2 Using the Duke treadmill score, patients may be divided into categories of low (score ≥ +5), intermediate (score < 5 but ≥ −10), and high risk (score < −10). The 5-year survival rates among patients categorized as low, intermediate, and high risk were initially reported at 97%, 91%, and 72%, respectively. The prognostic information derived from the Duke treadmill score is independent of coronary angiography findings. The predictive utility of the Duke score has been validated in many different subpopulations, including women. A low score is associated with a very low risk for cardiac death (0.3%–1.2% per year).
Stress Echocardiography
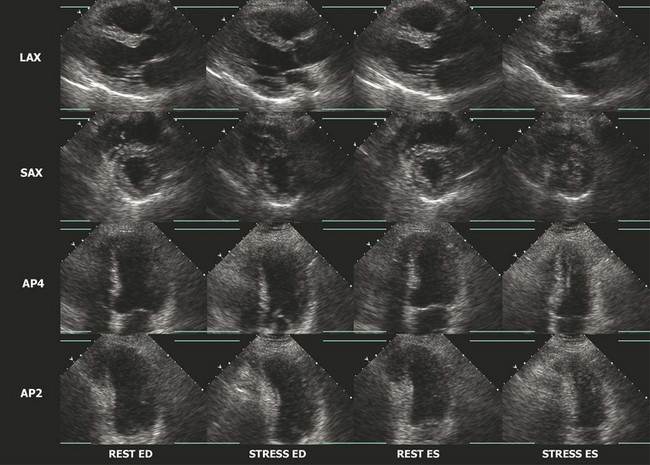
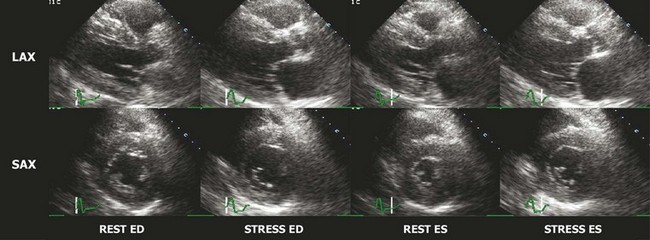
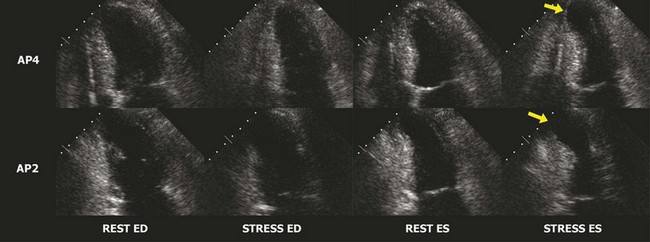
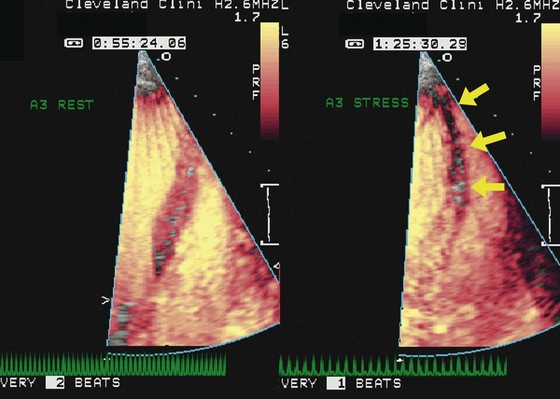
The interpretation of stress echocardiography is based on the identification of regional wall motion abnormalities induced by ischemia in the presence of obstructive CAD. The test has gained increasing acceptance following the introduction of digital acquisition, harmonic imaging, and contrast agents, all of which have incrementally contributed to increased image quality, reproducibility, and accuracy. The performance and interpretation of stress echocardiography require close supervision and attention to detail. Accordingly, accuracy varies significantly between experienced and inexperienced centers. Real time three-dimensional echocardiography facilitates faster data acquisition and better image segmentation and has been shown to improve diagnostic accuracy.3 Strain imaging has also been shown to improve the accuracy for detecting stress-induced ischemia and/or viability in dysfunctional segments.4 In stress echocardiography, regional wall motion is assessed from parasternal long, parasternal short, and apical images using a 17-segment model of the LV.5 Each segment is described as either normal, hypokinetic, akinetic, or dyskinetic, and the results of the individual segments are averaged to calculate a global wall motion score. The diagnosis of CAD is based on the detection of either resting or stress-induced regional wall motion abnormalities (Figs. 4-1, 4-2, and 4-3). In most cases, resting regional wall motion abnormality implies a prior myocardial infarction while a stress-induced regional wall motion abnormality implies ischemia caused by obstructive CAD. Stress echocardiography may also be used to evaluate the severity of ischemic mitral insufficiency.
Exercise Echocardiography
Resting and/or exercise-induced wall motion abnormalities may occur in patients with cardiomyopathies, microvascular disease, severe hypertension (increased afterload), or valvular disease; they are often a cause of false-positive interpretations. Several stress echocardiographic variables have been shown to have important prognostic value in patients with known or suspected CAD. A low exercise wall motion score index or a fall in exercise ejection fraction is highly predictive of an increased risk of adverse cardiac events. This prognostic value is equivalent to that of an MPI perfusion defect of >15%. Echocardiographic variables have incremental independent prognostic utility over other variables, such as the Duke Treadmill score.6 The rate of cardiac events in individuals with a normal exercise echocardiogram has been reported in several studies to be <1% per year.
Pharmacological Stress Echocardiography
Intravenous dobutamine, dipyridamole, or adenosine may be used as pharmacological stressors with echocardiography. Dobutamine is the most commonly used stressor in the United States. It is administered by continuous infusion at incremental rates starting from 5 and up to 50 mcg/kg per minute. It is often complemented by handgrip exercise and/or intravenous atropine (0.5–2.0 mg) to increase heart rate. Dobutamine increases myocardial oxygen demand by increasing contractility and heart rate. Adenosine and dipyridamole are used in many centers in Europe and South America. These agents induce ischemia by coronary steal. In order to induce regional wall motion abnormalities, the required doses are typically much higher than those used to provoke vasodilation during pharmacological MPI studies. The reported sensitivity and specificity of dobutamine echocardiography for the detection of obstructive CAD are equivalent to those reported for exercise echocardiography. The sensitivity is reduced in patients with concentric hypertrophy who experience cavity obliteration early during the test as well as in those who do not reach the target heart rate. Echocardiographic variables obtained during pharmacological stress have also been shown to have significant prognostic value.7 A normal dobutamine stress echocardiogram is associated with a low cardiac event rate in patients with suspected CAD and in those at clinically determined intermediate or high cardiac risk undergoing noncardiac surgery. The presence of stress-induced regional wall motion abnormalities, particularly when detected at low heart rates, is a strong predictor of cardiac events. Dobutamine stress echocardiography allows further risk stratification even in patients at intermediate or high risk who are receiving perioperative beta blockers.8 Dobutamine echocardiography may be performed for risk assessment in patients after myocardial infarction. In this setting, extensive resting regional wall motion abnormalities, stress-induced ischemia, absence of viability, and worsening left ventricular (LV) function with stress are associated with an increased risk of adverse events. In patients with ischemic heart disease and chronic LV dysfunction, dobutamine echocardiography is useful to identify myocardial viability. Improvement in regional contractility at lower rates of dobutamine (5–10 mcg/kg per minute) in segments that are akinetic or hypokinetic at rest predicts functional recovery after revascularization, particularly when those same segments exhibit a reduction in contractility at high dobutamine rates (biphasic response). Patients with ischemic LV dysfunction and viable myocardium who undergo revascularization have better outcomes than those who are not revascularized or have no evidence of viability regardless of revascularization. The sensitivity with which dobutamine echocardiography predicts recovery of function ranges between 74% and 88%; the specificity is between 73% and 87%. Compared with MPI, dobutamine stress echocardiography has higher specificity but lower sensitivity and overall similar accuracy for predicting functional recovery.9
Contrast Perfusion Imaging
Echocardiographic contrast agents consist of inert perfluorocarbon gases encapsulated in a biodegradable shell. Contrast microbubbles have a small diameter (<10 microns) that allows them to cross the pulmonary capillary bed. These agents are commercially available and approved for endocardial border definition in patients with suboptimal echocardiographic images. When they are exposed to ultrasound, these microbubbles act as strong reflectors owing to their liquid-gas interface. Contrast echocardiography may be used to evaluate myocardial perfusion. Since the LV myocardium has a dense capillary bed, the injection of contrast microbubbles results in myocardial enhancement proportional to the myocardial blood volume. During vasodilator stress in the presence of a flow-limiting stenosis, there is a reduction in capillary blood flow and myocardial blood volume in the segments supplied by the stenotic vessel. This may be detected as either a delay in myocardial enhancement following contrast injection or a relative reduction in enhancement in ischemic compared with normal segments (Fig. 4-4). Studies have shown relatively good agreement between myocardial contrast echocardiography and MPI for the detection of ischemia.10,11 An earlier study performed in high-risk patients but with normal wall motion at rest reported a sensitivity of 85% by myocardial contrast echocardiography versus 74% by MPI for the detection of obstructive CAD.12 The high spatial and temporal resolution of myocardial contrast echocardiography makes it suitable for the detection of nontransmural ischemia and milder ischemia where blood flow may be reduced but blood volume is preserved (late enhancement). However, data have been limited to a few reports and, in some studies where the sensitivity has been reported to be high, the specificity has been low. A previously published multicenter trial performed in 123 patients reported a sensitivity of 84% but a specificity of only 56%.13 Protocols for image acquisition and interpretation are considerably more technically demanding than those required for scintigraphic MPI. Thus, at present the use of contrast echocardiography for myocardial perfusion assessment is not an approved clinical indication in the United States.
Stress Scintigraphic Myocardial Perfusion Imaging
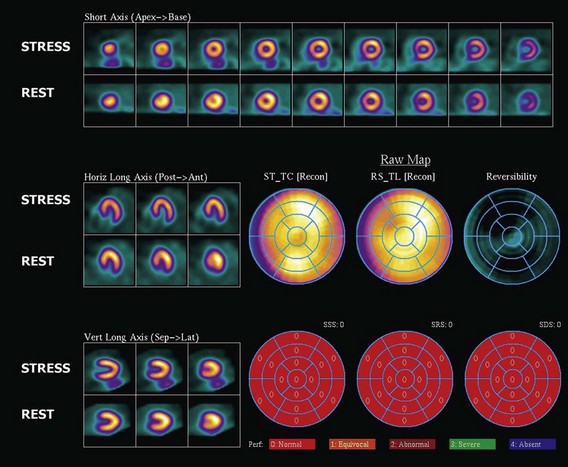
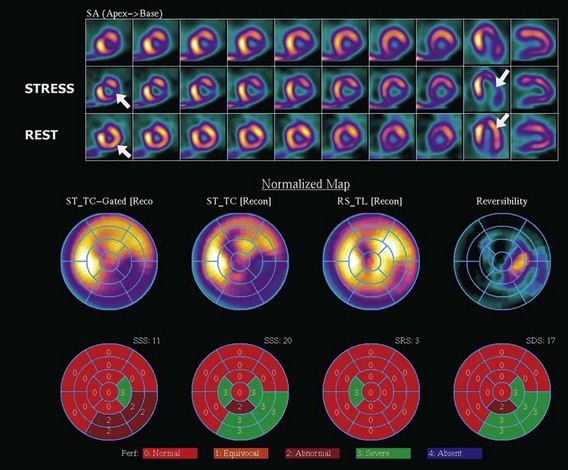
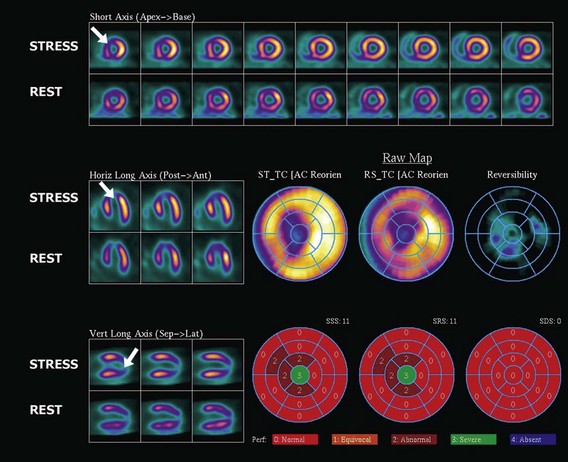
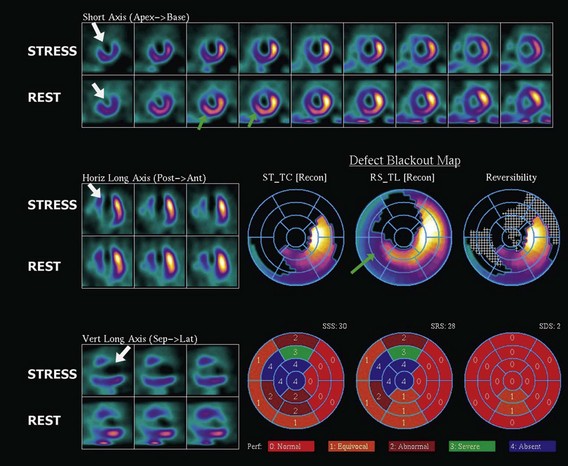
The assessment of myocardial perfusion imaging (MPI) by nuclear scintigraphic methods relies on the administration of a radionuclide isotope that is accumulated by the myocardium in proportion to blood flow. MPI is performed with either single-photon-emitting or dual-photon-emitting isotopes using single photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging systems, respectively. Thallium-201, technetium-99m sestamibi, and technetium-99m tetrofosmin are isotopes commercially available for SPECT imaging. Currently, technetium-99m-based isotopes are preferred for their higher photon energy, which results in higher image quality, and their shorter half-life, which results in lower radiation exposure. These isotopes emit single photons that travel through tissues and must be detected on a photon-sensitive detector. The direction of the traveling photon is determined by adding a lead collimator, which acts as an x-ray filter between the source and the detector. This collimator rejects most of the photons not traveling along certain directions; therefore only a percentage of the emitted photons are used for imaging. Spatial resolution is given by the space between the bars in the collimator. Increasing spatial resolution improves image quality but requires higher rejection of photons, thus reducing efficiency and increasing radiation exposure to the patient. Most dual-photon-emitting isotopes are produced using a cyclotron and have a very short half-life. These isotopes decay with the emission of a positron; after a series of collisions with atomic electrons from the tissues, it annihilates with a nearby electron and produces two high-energy photons emitted in opposite directions. A PET system relies on the simultaneous detection of the photons. These photons travel toward detectors positioned around the subject, where they interact, becoming absorbed and producing an electrical signal. The detector signals are processed by specialized coincidence circuitry. If the difference in the time of arrival of these photons is smaller than a predetermined value (typically <10 ns) a signal is recorded. Unlike SPECT imaging, PET does not require collimation, since the position of the emitting target is determined by the simultaneous registration of the two photons traveling at 180 degrees. Thus, the efficiency of PET is several magnitudes greater, providing higher resolution, lower noise, and lower radiation exposure. The signals recorded are used to reconstruct a three-dimensional image. The spatial resolution of PET images is closely related to the physical size of the detector elements and has dramatically increased in recent times with the introduction of time-of-flight (TOF) technology (Fig. 4-5). With either SPECT or PET cardiac perfusion studies, images are obtained after stress and at rest. For segmentation of the LV, a 17-segment model is applied. Images are interpreted visually or by using automated quantification based on normalized data. Myocardial scar is determined by the presence of a relative perfusion defect (compared with the segment with highest counts), which persists on both stress and resting images. Ischemia is determined by the presence of a perfusion defect on stress images that improves or resolves on the resting images (Figs. 4-6, 4-7, 4-8, and 4-9).