Primary Aortoenteric Fistulae
CARLA C. MOREIRA and JAMES T. MCPHEE
Presentation
A 74-year-old male active smoker with a history of chronic obstructive pulmonary disease (COPD), hypertension, and hyperlipidemia complains of 1 week of malaise, low-grade fevers, and chills. He has a family history of a father with an “aneurysm” and a brother who died suddenly at age 70. He presents to the emergency department after an episode of vomiting bright red blood twice at home. He denies abdominal pain, back pain, flank pain, or dysuria. Physical examination is notable for temperature of 99.9°C, heart rate of 115 bpm, blood pressure of 110/80 mm Hg and 90/70 mm Hg on right and left arms, respectively. He is somewhat pallid in appearance and has mild mid-abdominal tenderness without guarding or rebound, and a pulsatile abdominal mass is found. He has 2+ femoral pulses bilaterally. Electrocardiogram (EKG) and chest x-ray (CXR) are unremarkable. Gastroenterology consultants perform an esophagogastroduodenoscopy (EGD) through the second portion of the duodenum and find no evidence of gastritis, peptic ulcer disease (PUD), or hemobilia. A nasogastric tube (NGT) is left in place. Next, a computed tomography (CT) angiogram is performed.
Differential Diagnosis
The differential diagnosis for upper gastrointestinal (GI) bleed includes PUD, gastritis, esophageal varices, esophagitis, gastritis, neoplasms, hemobilia, and aortoenteric fistula (AEF).
Case Continued
CT angiogram demonstrates a 5.2-cm infrarenal abdominal aortic aneurysm (AAA) (Fig. 1). Concern is raised for primary AEF due to air bubbles within the aortic wall and within the mural thrombus. Additionally, stranding of the retroperitoneum and loss of the fat plane between the fourth portion of the duodenum and the aortic wall are noted. Intravenous (IV) access is obtained, and a rapid blood sample is sent to the blood bank. The patient has ongoing bleeding from the NGT and receives two units of packed red blood cells (PRBC).
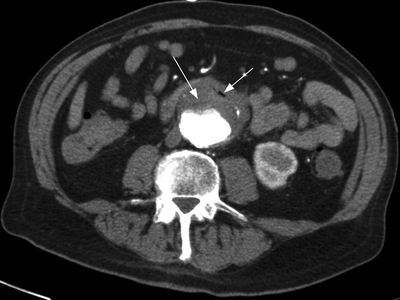
FIGURE 1 CT angiogram with air within the aortic thrombus, highly suggestive of aorto-enteric fistulae.
Diagnosis
Despite technologic advances, the cornerstone of diagnosing AEF continues to be high index of clinical suspicion. The classic triad of GI bleeding, sepsis, and abdominal pain is rarely found in a single patient. The initial bleeding is usually minor and self-limiting. Additionally, the interval between this “herald bleed” and massive subsequent rebleeding is unpredictable, occurring hours to months later, and is a major cause of delay in making the definitive diagnosis.
CT scanning has been advocated as the preferred initial diagnostic test for patients with AEF, with a reported sensitivity of up to 93% in some series. The presence of a pulsatile abdominal mass, fever, hematemesis, and air within the wall of an aneurysmal aorta are indicative of primary AEF. The diagnosis can be confirmed by EGD performed by an experienced endoscopist who is able to examine the entire duodenum and localize the small defect in the wall with a 25% to 83% success rate. Angiography should be reserved for patients in whom the diagnosis of AEF is unclear to help determine a source of bleeding and aid in planning arterial reconstruction.
Recommendation
The patient is prepared for emergent surgery. Adequate IV access is obtained, resuscitation efforts are undertaken and communication with the blood bank regarding the potential for massive transfusion is initiated. Broad-spectrum antibiotics are initiated as well, and the patient is monitored in appropriate setting, commonly the intensive care unit (ICU). A frank discussion with the patient and family regarding the grave nature of the condition will appropriately set the stage for a likely complicated postoperative course with high rates of major morbidity and mortality.
On occasion, appropriately informed patients and caregivers may not be desirous of major complex surgery and/or may be of prohibitive risk (advanced age, advanced cancer, or severe comorbidity) and may opt for comfort measures only. Preoperative cardiology assessment is unnecessary in this setting, and the surgeon and anesthesiologist may proceed if they deem the patient to be of acceptable risk. Preoperative bilateral arm blood pressures should be performed, as well as a thorough lower extremity pulse exam. The surgical approach will be dictated by the patient’s hemodynamic stability, aortic aneurysm anatomy (infrarenal, juxtarenal, or pararenal), conduit availability, and surgeon preference. The tenets of repair are proximal aortic control, debridement of nonviable or infected tissue, intestinal repair, and establishment of distal perfusion by either in-line reconstruction or extra-anatomic means.
Surgical Approach
The surgical strategy used in the treatment of AEF depends primarily on two things, whether the patient is hemodynamically stable at the time of surgery and whether infection is present in the aortic bed. In an actively bleeding, unstable patient, expeditious aortic control is imperative. The abdomen is usually explored through midline incision, allowing for full exposure and examination of the intestine and aorta. Supraceliac aortic control should be established by either isolating the aorta through the crus of the diaphragm or by medial visceral rotation. Left retroperitoneal approach has been advocated in cases where the proximal aorta may be difficult to access because of a short infrarenal neck. However, the major disadvantage of this approach is that it offers less favorable exposure of the duodenum and limited exposure of the right iliac artery.
After establishing proximal control, the abdomen should be carefully explored and distal control established by clamping of the iliac vessels or by means of occlusion balloon catheters. Careful dissection of the adherent bowel to the diseased aorta allows proper identification of the fistula. Gross spillage from the bowel can be quickly temporized and bowel repair deferred until after the aortic pathology is addressed using the strategies discussed in detail below. The enteric fistula can be treated with a simple primary repair by transverse closure, resection and anastomosis, or Roux-en-Y reconstruction depending on anatomic limitations. Gastrostomy and feeding jejunostomy tubes are generally placed to aid in postoperative feeding, as these patients usually have a prolonged postoperative course.
Extra-Anatomic Bypass
The most expeditious means of accomplishing revascularization is extra-anatomic bypass through uninfected tissue. Axillobifemoral bypass is the option of choice if the infected AAA is truly infrarenal, as oversewing of the aortic stump should not impinge upon the renal arteries. This can be performed in a staged manner, depending on the hemodynamic stability of the patient.
In a two-stage procedure, the diagnosis of AEF has been made in a stable patient who is not actively bleeding. During the first operation, which can be the same day or a day before, a standard axillobifemoral bypass is performed using externally supported (ringed) polytetrafluoroethylene (PTFE). If the infection has extended to the femoral arteries, which would be quite rare for primary AEF, bilateral axillounifemoral grafts tunneled laterally to the available superficial femoral, profunda femoris, or popliteal arteries may be required. At the second operation, laparotomy, aneurysmectomy with debridement of aorta and infected tissue, intraoperative cultures are sent, and two-layer aortic closure and omental buttress of aortic stump are performed.
Alternatively, in an unstable or bleeding patient, this is performed as a one-stage procedure. The first step entails laparotomy, debridement, aortic and bowel repair, and abdominal closure. The patient is then reprepped and redraped, and new set of instruments are used for the axillobifemoral bypass. Tunneling of the left extra-anatomic bypass may be problematic if a left retroperitoneal approach was used. A proposed solution is the use of composite graft; the synthetic extra-anatomic graft is tunneled in noncontaminated tissue plane, and an autogenous graft is tunneled in potentially contaminated field. Care must be taken to avoid contamination of the synthetic component. More commonly, a right axillobifemoral bypass will be used, specifically in this case where the right arm pressure was 20 mm Hg higher than the left.
The operating surgeon should be familiar with techniques, such as rotation of a sartorius muscle flap, to provide adequate soft tissue coverage of the femoral vessels. The biggest risk associated with this technique is stump blow-out, especially if tissue margins are positive for bacteria. Appropriate antibiotic coverage is important to minimize the risk of seeding the extra-anatomic bypass graft, which can occur in approximately 15% to 25% of patients. The advantage of preemptive extra-anatomic bypass is the elimination of lower extremity ischemia and decreasing risk of subsequent amputations in these patients.
In-line Reconstruction
Primary AEF is a distinct and separate clinical entity than secondary AEF. In the absence of gross evidence of infection, some authors have advocated repair of the duodenum and in situ aortic reconstruction with a standard synthetic prosthesis in selected patients. The involved aorta and surrounding tissues are debrided. Care must be taken to avoid contamination by intestinal content at the time of surgery to minimize risk of infecting the aortic prosthesis. Well-vascularized, healthy tissue should be positioned between the repaired bowel and the prosthetic graft. This approach obviously requires sound clinical judgment. The same authors advocating this treatment strategy have also advocated close follow-up and long-term antibiotics because up to 30% of aneurysms associated with primary AEF formation may prove to be infected.
Some recent studies have shown promising results with variety of conduit options, such as antibiotic-bonded/soaked synthetic grafts (i.e., rifampin-soaked and silver-coated Dacron grafts), as a reasonable option for treatment of abdominal aortic infections. Cryopreserved arterial homograft (CAH) is also considered a suitable conduit in the setting of arterial infection and has shown favorable outcomes even against highly virulent microorganisms. The availability of these grafts and the relatively low rates of secondary procedures have been listed as their important advantages. Again, careful patient selection and clinical judgment are paramount if these treatment options are undertaken. These conduits may be associated with early and late complications, such as aneurysm degeneration, occlusion, and reinfection.
Neo-Aortoiliac System
Clagett et al. described an innovative approach to revascularization of the lower extremities by using the femoral veins to reconstruct an autogenous neo-aortoiliac system (NAIS) in the setting of an infected aortic prosthesis. A similar approach may be considered in the setting of primary aortic infection. This approach allows in situ vascular reconstruction with completely autologous tissue, and it has been shown to be effective and durable. The primary disadvantage of this procedure is that its time consuming, which can be problematic in the setting of hemorrhage, hemodynamic instability, and prolonged lower extremity ischemia. An additional consideration is the risk of postoperative leg swelling. In selected patients, this situation may be ameliorated if multiple surgical teams are available to permit simultaneous vein harvest. Experience with the NAIS procedure in the setting of primary AEF is limited.
Endovascular Stent Graft “Bridging”
The use of endovascular stent grafts in secondary AEF, where a new stent graft is deployed into a previously placed stent graft, has been described as a quick method to control bleeding and facilitate aortic control by several authors. However, this method is not typically a viable long-term option for two main reasons: (1) the enteric fistula is not repaired leading to persistent infection and possibly bleeding from the injured bowel mucosa and (2) aortic and retroperitoneal debridement is not possible, again leading to chronic infection and eventual contamination of the newly placed stent graft. Another limitation is that standard anatomic endograft restrictions apply, thus an adequate infrarenal seal zone of healthy aorta as well as adequately sized access vessels are necessary to consider this temporizing technique. Currently, this technique should be reserved for life-and-death scenarios to quickly to address ongoing hemorrhage. Still, some authors advocate the use of this procedure as a “bridge to surgery”; either carried out simultaneously with preparation for laparotomy or in a delayed fashion with semielective graft explant and extra-anatomic bypass in a timely fashion (Table 1).
TABLE 1. Primary AEF

AEF, aortoenteric fistula; GIB, gastrointestinal bleed; AAA, abdominal aortic aneurysm; OR, operating room.



