Chapter 31 Prevention and Treatment of Stroke
Acute ischemic stroke (AIS) occurs after occlusion of an intracranial or extracranial vessel by a thrombus that in most cases has embolized from the heart or a more proximal vessel. Unlike acute myocardial infarction (AMI), in situ thrombosis is uncommon. As a consequence of acute vascular occlusion, a cascade of intracellular events (Fig. 31-1) is initiated; over varying periods of time, they lead to irreversible tissue injury such as infarction.1 The temporal development of infarction within the ischemic brain region is quite variable, and portions of the ischemic brain tissue may not be irreversibly injured for many hours after the initial vascular occlusion.2 Ischemic brain tissue that remains viable and potentially amenable to salvage with timely initiation of therapeutic interventions is called the ischemic penumbra, and this potentially salvageable tissue is the target of AIS therapies.3,4 The basic concept underlying AIS therapy is that reducing the extent of brain infarction should translate into improved clinical outcome, as measured by commonly used outcome scales such as the modified Rankin Scale (mRS) or Barthel Index.5
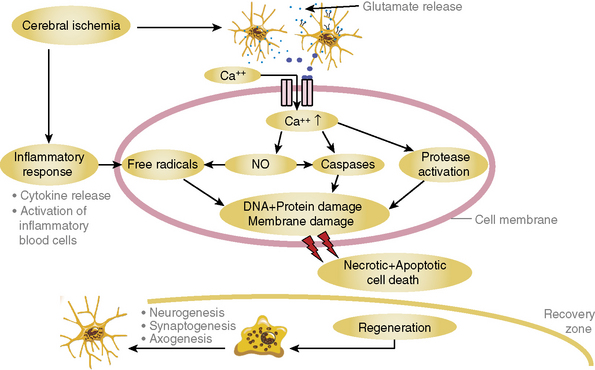
Figure 31-1 Depiction of the major events that encompass the ischemic cascade of cellular injury.
DNA, deoxyribonucleic acid; NO, nitric oxide.
(Courtesy Dr. Wolf-Rudiger Schaebitz.)
The most important factor predisposing ischemic brain tissue to infarction is the severity of cerebral blood flow (CBF) decline.6 Regions with little or no residual CBF will evolve into infarction rapidly and are not the target of AIS therapies because reperfusion cannot in most cases be performed rapidly enough to salvage this ischemic core region. In the ischemic penumbra, CBF decline is more modest, and this ischemic tissue progresses more slowly toward infarction, providing a time window for intervention that can salvage tissue to some extent. A variety of definitions for the ischemic penumbra were suggested over time and are outlined in Box 31-1. Besides the severity of CBF decline, other factors that affect evolution of ischemic injury include temperature, glucose, blood pressure, and other metabolic factors.7,8 The implication of these factors that contribute to the evolution of ischemic injury is that individual AIS patients have quite variable therapeutic time windows for successful therapeutic intervention, and that the earlier a therapy is initiated, the more likely it is to be beneficial. Acute ischemic stroke therapy can be divided into two broad areas: (1) recanalization/reperfusion approaches directed at improving altered CBF within ischemic tissue and (2) neuroprotection designed to impede the cellular consequences of ischemic injury. The focus of this chapter will be on the former because no neuroprotection strategies have been demonstrated to be of significant benefit. Recanalization/reperfusion can be accomplished with intravenous (IV) or intraarterial (IA) thrombolytics as well as mechanical devices. These approaches comprise the currently available AIS treatments.
![]() Box 31-1 Definitions of the Ischemic Penumbra Over Time
Box 31-1 Definitions of the Ischemic Penumbra Over Time
 A region of reduced CBF with absent electrical activity but preserved ion homeostasis and transmembrane electrical potentials
A region of reduced CBF with absent electrical activity but preserved ion homeostasis and transmembrane electrical potentials
 A region with reduced CBF and preserved energy metabolism
A region with reduced CBF and preserved energy metabolism
 A region with impaired protein synthesis but preserved ATP levels
A region with impaired protein synthesis but preserved ATP levels
 A region that is potentially salvageable with timely intervention*
A region that is potentially salvageable with timely intervention*
ATP, adenosine triphosphate; CBF, cerebral blood flow.
Prehospital and Emergency Department Management of Ischemic Stroke
Prehospital management and field treatment are critically important to increasing survival rates of stroke patients. This phase starts with the emergency medical services (EMS) call and continues in the hospital emergency department (ED; Table 31-1). The majority of ischemic stroke patients do not reach the hospital soon enough, owing to lack of local services, facilities, and social reasons. When first suspected to have a stroke, the patient should be rapidly transported to an appropriate facility for diagnostic evaluation and treatment initiation.9 Stroke patients who present within 3 to 4.5 hours of symptom onset are eligible for IV thrombolysis.10–14 Emergency medical services use is strongly associated with a decreased time to initial physician examination, initial computed tomography (CT) imaging, and neurological evaluation.13,15–17 The benefits of EMS contact are superior to contacting the family physician or hospital directly, and were confirmed with several studies.18,19 Stroke should be given a priority dispatch as for MI and trauma.20 Patients who show signs and symptoms of hyperacute stroke must be treated as time-sensitive emergency cases and transported without delay to the closest institution that provides emergency stroke care.
Table 31-1 Stroke Chain of Survival
| Detection | Recognition Of Stroke Signs And Symptoms |
|---|---|
| Dispatch | Call 911 (emergency phone number) and priority EMS dispatch |
| Delivery | Prompt transport and prehospital notification to hospital |
| Door | Immediate ED triage |
| Data | ED evaluation, prompt laboratory studies, and CT imaging |
| Decision | Diagnosis and decision about appropriate therapy |
| Drug | Administration of appropriate drugs or other interventions |
CT, computed tomography; ED, emergency department; EMS, emergency medical services.
Adapted from Adams HP Jr, del Zoppo G, Alberts MJ, et al: Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 38:1655–1711, 2007.9
To ease and facilitate this process, medical authorities and media sources should encourage the recognition of stroke signs by providing public education about this condition.21 All members of the public should be able to recognize and identify the signs and symptoms of stroke, which include sudden localized weakness, difficulty speaking, loss of vision, headache, and dizziness.22 Patient, family, and caregiver education is an integral part of stroke care that should be addressed at all stages across the continuum of stroke care for both adult and pediatric patients.22 Currently, thrombolytic treatment with tissue plasminogen activator (tPA) is the only approved treatment option for AIS. The National Institute of Neurological Disorders and Stroke (NINDS) and Advanced Cardiac Life Support Resources (ACLSR) recommend the possible timing sequences shown in Table 31-2 for the potential thrombolysis candidate.
Table 31-2 Stroke Evaluation Time Benchmarks for Potential Thrombolysis Candidate
| Time Interval | Time Target |
|---|---|
| Door to doctor | 10 min |
| Access to neurological expertise | 15 min |
| Door to CT scan completion | 25 min |
| Door to CT scan interpretation | 45 min |
| Door to treatment | 60 min |
| Admission to monitored bed | 3 h |
CT, computed tomography.
Data from the Thomas Lewis Latané (TLL) Temple Foundation Stroke Project controlled trial showed the benefits of educational interventions on stroke identification and management targeting patients, EMS, hospitals, and community physicians. This approach increased thrombolytic use in patients with ischemic stroke from 2.21% to 8.65% as compared with communities that did not have such programs, which saw only a 0.06% increase. For patients with ischemic stroke who were eligible for thrombolytic therapy, rates of tPA usage increased from 14% to 52% in intervention communities.23,24 Prehospital delays continue to contribute the largest proportion of time to late initiation of therapy.25
Emergency medical services arrival starts the diagnostic and management processes. The EMS crew should transfer the patient to a medical center that can provide appropriate diagnostic and treatment modalities to stroke patients.9 After the ambulance arrives on the scene, EMS providers should obtain a brief history and patient examination, stabilize vital signs, and rapidly transport the patient to the closest, most appropriate facility (Table 31-3). Prehospital evaluation is helpful for ED physicians and the inpatient care team for planning treatment options. The Los Angeles Prehospital Stroke Screen and Cincinnati Prehospital Stroke Scale are the most widely used and preferred prehospital evaluation instruments and facilitate evaluation of potential stroke patients. Critical medical interventions in the ED should focus on the need for intubation, blood pressure control, and determining risk/benefit for thrombolytic intervention.26 General ED stroke care issues are outlined in Table 31-4.27,28
Table 31-3 Guidelines for Emergency Medical Services Management of Patients with Suspected Stroke
| Recommended | Not Recommended |
|---|---|
| Manage ABCs | Dextrose-containing fluids in nonhypoglycemic patients |
| Cardiac monitoring | Hypotension/excessive blood pressure reduction |
| IV access | Excessive IV fluids |
| Oxygen (as required for O2 saturation <92%) | |
| Assess for hypoglycemia | |
| Nil per os (NPO) | |
| Alert receiving emergency department | |
| Rapid transport to closest appropriate facility capable of treating acute stroke |
ABCs, airway, breathing, circulation; IV, intravenous.
Adapted from Adams HP Jr, del Zoppo G, Alberts MJ, et al: Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 38:1655–1711, 2007.9
Table 31-4 General Management of Patients with Acute Stroke
| Blood Glucose | Treat Hypoglycemia With D50 |
|---|---|
| Blood pressure | Evaluate recommendations for thrombolysis candidates and noncandidates |
| Cardiac monitor | Continuous monitoring for ischemic changes and atrial fibrillation |
| IV fluids | Avoid D5W and excessive fluid administration; IV isotonic sodium chloride solution at 50 mL/h unless otherwise indicated |
| Oral intake | NPO initially; aspiration risk is great; avoid oral intake until swallowing assessed |
| Oxygen | Supplement if indicated (SaO2 <93%, hypotensive, etc.) |
| Temperature | Avoid hyperthermia; oral or rectal acetaminophen and cooling blankets as needed |
IV, intravenous; NPO, nil per os; SAO2, arterial blood oxygen saturation.
Adapted from Krieger D, Hacke W: The intensive care of the stroke patient. In Barnett HJ, Mohr JP, Stein BM, editors: Stroke: pathophysiology, diagnosis and management, ed 3, New York, 1998, Churchill Livingstone; and SPORTIF Executive Steering Committee for the SPORTIF V Investigators: Ximelagatran vs. warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomized trial. JAMA 293:690–698, 2005.27,28
Acute stroke patients urgently need IV access and cardiac monitoring in the ED, preferably initiated in the transporting ambulance. These patients are at also risk for acute cardiac diseases such as arrhythmias and myocardial infarction (MI). In addition, atrial fibrillation may be associated with acute stroke as either the etiology (embolic disease) or as a result.29,30 Acute stroke patient evaluation in the ED should include a detailed history, physical examination, neurological examination, and stroke scale scores (National Institutes of Health Stroke Scale [NIHSS] and appropriate diagnostic tests; Box 31-2).9
![]() Box 31-2 Immediate Diagnostic Studies for Patients with Suspected Acute Ischemic Stroke*
Box 31-2 Immediate Diagnostic Studies for Patients with Suspected Acute Ischemic Stroke*
Selected Patients
Arterial blood gas tests (if hypoxia is suspected)
Chest radiography (if lung disease is suspected)
Lumbar puncture (if SAH is suspected and CT scan is negative for blood)
Adapted from Adams HP Jr, del Zoppo G, Alberts MJ, et al: Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 38:1655–1711, 2007.9
* Although it is desirable to know the results of these tests before giving rtPA, thrombolytic therapy should not be delayed while awaiting the results unless (1) there is clinical suspicion of a bleeding abnormality or thrombocytopenia, (2) the patient has received heparin or warfarin, or (3) use of anticoagulants is not known.
Patients presenting with compromised ventilation require emergent airway control via nasal oxygenation or rapid sequence intubation. Adequate tissue oxygenation is important in the acute management of acute cerebral ischemia to prevent hypoxia and further brain damage. The most common causes of hypoxia in the patient with acute stroke are partial airway obstruction, hypoventilation, atelectasis, or aspiration pneumonia.9,26,27,31 Oxygen need should be monitored with pulse oximetry, with a target oxygen saturation level of 92% or better.32 Endotracheal intubation, supplemental nasal oxygen, and hyperbaric oxygen are choices to augment oxygen intake. If brain herniation is present, hyperventilation using mechanical ventilation is an option to decrease intracranial pressure (ICP) by decreasing CBF, is recommended with an endpoint arterial partial pressure of carbon dioxide (PCO2) of 32 to 36 mmHg. Intravenous mannitol can be considered as well to reduce increased intracranial pressure. Oxygen supplementation should be guided by a pulse oximeter.26,27,31
Acute Stroke Therapy
The only AIS treatment currently approved by regulatory agencies is IV tPA initiated within 3 hours after stroke onset. Approval of this treatment was based on results of the NINDS tPA trial that demonstrated a highly significant improvement in 90-day outcome on various outcome measures including mRS, Barthel Index, and NIHSS.33 The benefit was observed in different stroke subtypes and in patients with various ranges of baseline stroke severity, ranging from relatively mild to fairly severe.34 Despite a risk of 6.4% for symptomatic intracranial hemorrhage with IV tPA treatment observed in the NINDS trial, the overall treatment effect was highly significant. Following the NINDS trial, several other studies of IV tPA were performed that tried to extend the therapeutic time window to 6 hours from stroke onset.35–37 None of these demonstrated a significant benefit on the primary outcome measure chosen for the trial. However, a combined analysis of the European Cooperative Acute Stroke Studies (ECASS) I and II as well as the ATLANTIS trial demonstrated a significant treatment effect with IV tPA out to 4.5 hours from stroke onset.38 This observation suggested that the therapeutic time window for IV tPA could be extended and was evaluated in the ECASS III trial. The study evaluated AIS patients between 3 and 4.5 hours after onset and reflected the European license for tPA: excluding patients over 80, those with very severe strokes, history of prior stroke, diabetes, and use of anticoagulants prior to stroke.39 The ECASS III study demonstrated that 52.4% of tPA-treated patients achieved a favorable outcome of 0 to 1 on the mRS, compared to 45.2% with placebo treatment (odds ratio [OR], 1.34; 95% confidence interval [CI], 1.02-1.76; P = 0.04). The results of ECASS III led to recommendations from the American Heart Association (AHA) and other groups that the use of IV tPA be extended to 4.5 hours in selected AIS patients.40 Subgroup analysis of the ECASS III data suggested that patients older than 65 and those with more severe strokes had less benefit than younger patients or those with milder deficits41 (Box 31-3). Unfortunately, only standard CT scans were employed in the ECASS III trial, so it is unclear how more modern imaging techniques such as diffusion/perfusion magnetic resonance imaging (MRI) or perfusion CT may contribute to identifying AIS patients more or less likely to benefit from IV tPA in this extended time window when ischemic penumbral survival tends to wane.
![]() Box 31-3 Subgroup Analysis of the ECASS III Trial of Intravenous tPA in the 3- to 4.5-Hour Time Window
Box 31-3 Subgroup Analysis of the ECASS III Trial of Intravenous tPA in the 3- to 4.5-Hour Time Window
Patient Characteristics Associated with Modest or No Benefit with tPA
Adapted from Bluhmki E, Chamorro A, Dávalos A, et al: Stroke treatment with alteplase given 3.0-4.5 h after onset of ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol 8:1095–1102, 2009.42
Currently, IA thrombolysis remains an unproven therapy for AIS patients, despite its widespread use at larger centers. The only phase III trial that evaluated this therapeutic approach was the PROACT-III trial that compared prourokinase (n = 121) to placebo (n = 59) in severely compromised patients with angiographically confirmed large-vessel occlusion treated up to 6 hours after stroke onset.42 A prior safety trial demonstrated a reasonable safety profile. In the PROACT-II trial, 40% of treated patients achieved a favorable outcome, defined as an mRS score of 0 to 2, compared to 25% in the placebo group (P = 0.04). The rates of partial or complete recanalization were 66% and 18%, respectively. Symptomatic ICH was increased in the prourokinase group, but not significantly. The trial showed that IA therapy could be beneficial up to 6 hours from stroke onset in patients with large intracranial vascular occlusions. Prourokinase was not approved by regulatory agencies based upon this one modestly sized trial, and a second confirmatory trial was never performed. Prourokinase is not available for clinical use.
Studies of the IA use of tPA are limited to combination trials with initial IV tPA use followed by subsequent IA treatment. In the IMS I trial, the combined use of tPA both IV and IA was observed to carry a similar risk for ICH as IV tPA alone in the NINDS IV trial.43 In the IMS II trial, patients initially received IV tPA within 3 hours of stroke onset. An angiogram was then performed, and if a clot was observed, IA therapy was initiated either through a standard catheter or an EKOS microinfusion catheter.44 Among 81 patients included in the trial, 26 received IV tPA alone, 33 IV plus IA tPA via the EKOS catheter, and 19 the combination IA via a standard catheter. There was no control group, but when compared to the placebo group in the NINDS trial, this combined treatment approach tended to have a more favorable outcome. IMS III, a much larger trial comparing IV tPA with various combined IV and IA therapies, is ongoing, but recruitment has lagged. A European study comparing IV versus IA tPA is also ongoing.
The use of urokinase given IA was studied in the Japanese MELT trial that randomized 114 patients with angiographically confirmed middle cerebral artery (MCA) occlusion to active therapy or standard medical therapy within 6 hours of stroke onset.45 The IA urokinase group achieved a favorable outcome (mRS 0-2) in 49.1% of patients, compared to 38.6% in the control group, a nonsignificant trend toward benefit in an underpowered trial. A detailed analysis of the randomized IA thrombolytic trials concluded that there was no evidence of proven benefit with this treatment approach when compared with the expected rate in a prognostic model.46
Device recanalization was evaluated with both the MERCI retriever and PENUMBRA device. Both of these devices received U.S. Food and Drug Administration (FDA) clearance to remove clots in intracranial blood vessels but are not approved to treat AIS, a curious paradox when considering why a device would be used to remove a clot. Approval of the MERCI retriever was based upon an open nonrandomized trial in 141 AIS patients, most with anterior circulation occlusions, treated up to 8 hours after stroke onset who did not receive tPA.47 These treated patients were then compared to historical controls, the placebo group from the PROACT-II trial. Partial or complete recanalization occurred in 48% of the patients treated with the MERCI retriever, but only 27.1% achieved an mRS score of 0 to 2 at 90 days, essentially the same as the 25% rate of mRS observed in the control group in the PROACT-II trial. Serious adverse events related to employment of the device occurred in 7.1% of the patients, but this did not include an additional 7.8% of patients who developed symptomatic ICH.
A second open-label study of a subsequent generation of the MERCI retriever, the Multi-MERCI study, was reported, but this study is difficult to interpret because concomitant use of IV and/or IA tPA was allowed and occurred in the majority of the patients in the study.48 The partial or complete recanalization rate was slightly higher (57.3%), as was the rate of patients achieving a 90-day mRS score of 0 to 2 (36%). Symptomatic ICH occurred in 9.8% of patients, and serious adverse events occurred in 7.9%. The PENUMBRA device was also evaluated in an open trial and compared to historical controls from the PROACT II trial.49 In this study, 125 patients were treated with the device up to 8 hours after stroke onset. Partial or complete recanalization occurred in 82% of the patients, device-related serious adverse events in 1.6%, and symptomatic ICH in 11.2%. At day 90, 25% of the patients achieved an mRS of 0 to 2, identical to the control group in the PROACT II trial.
In addition to recanalization therapies that have demonstrated clear benefit when initiated within 4.5 hours of stroke onset, the only other acute intervention with a documented significant improvement in outcome is the use of specialized stroke care units. In acute coronary care, specialized units have a long-standing history and proven track record. For AIS, the development and use of specialized care units is much more recent, and efficacy was documented later than for coronary care units. Several studies found that admission of AIS patients to stroke care units reduces mortality and disability when compared to care on general medical units with stroke team consultation.50,51 The precise reasons for these benefits are uncertain but likely relate to stricter adherence to care guidelines, better blood pressure and glucose management, and earlier mobilization.52 Additionally, it is now apparent that management guidelines such as the AHA “Get with the Guidelines” recommendations reduce complications after AIS, and that these guidelines should be implemented both in stroke units and general hospital units if at all possible.53
The other approach to AIS treatment besides recanalization/reperfusion is neuroprotection, or reduction of infarction by treatments targeting the manifold cellular consequences of focal brain ischemia, which has a long and undistinguished track record. In animal stroke models, many categories of neuroprotective drugs reduce infarct size, and some also improve functional outcome54,55 (Box 31-4). The reduction of infarct size for many neuroprotective drugs occurred without reperfusion, implying that enough of the drug reached the ischemic penumbra in sufficient concentration to impede development of infarction. This can occur because there is sufficient residual CBF in the penumbral region to deliver the neuroprotective drug and allow this tissue to survive.
Adapted from Schabitz WR, Fisher M: Perspectives on neuroprotective stroke therapy. Biochem Soc Trans 34:1271–1276, 2006; and Donnan GA: A new road map for neuroprotection. Stroke 39:242–248, 2008.55,56
Based on preclinical data of varying quality and extensiveness, many neuroprotective drugs went into clinical development, including phase III trials in some cases.56 None of the neuroprotective drugs demonstrated significant efficacy, and currently no neuroprotective drug is available for AIS treatment. Many reasons for the multitude of neuroprotective drug development programs were proposed regarding both the preclinical assessment of these agents and the clinical development programs57 (Box 31-5). Neuroprotection as an AIS treatment strategy remains appealing either as monotherapy or in combination with reperfusion. A safe and modestly effective neuroprotective drug with clear stand-alone efficacy for improving outcome after AIS could be widely used, especially in hospitals without the adequate infrastructure for giving even IV tPA. Combining neuroprotection with reperfusion therapy can be envisioned in several ways. Very early initiation of a neuroprotectant could potentially extend survival of the ischemic penumbra, allowing for later deployment of an IV or IA reperfusion therapy. In animals, both high-flow 100% oxygen delivery and granulocyte colony stimulating factor (GCSF) have the capability to extend penumbral survival, but this approach has not yet been tested in clinical trials.58,59 Another potential combination of neuroprotection with reperfusion would be to use an appropriate agent to reduce potential deleterious tissue consequences of successful reperfusion (i.e., reperfusion injury).60 Neuroprotective drugs that affect free radicals or reduce the recruitment of inflammatory white blood cells (WBCs) may be particularly suited to reduce reperfusion injury, and clinical development programs with such agents after successful reperfusion should be considered.
Preclinical Testing Flaws
 Inadequate sample sizes in treatment experiments
Inadequate sample sizes in treatment experiments
 Lack of adequate physiological monitoring
Lack of adequate physiological monitoring
 Lack of blinding to treatment outcomes
Lack of blinding to treatment outcomes
 Not testing the drug in animals that were female, aged, or had relevant comorbidities
Not testing the drug in animals that were female, aged, or had relevant comorbidities
 Only testing the study drug in rodents
Only testing the study drug in rodents
 Only evaluating histological endpoints and not behavioral outcomes
Only evaluating histological endpoints and not behavioral outcomes
 Lack of determining effects on both ischemic gray and white matter
Lack of determining effects on both ischemic gray and white matter
Clinical Trial Flaws
 Treating patients too long after stroke onset
Treating patients too long after stroke onset
 Including stroke subtypes not likely to respond to treatment, such as lacunar stroke patients
Including stroke subtypes not likely to respond to treatment, such as lacunar stroke patients
 Moving to phase III trials without adequate assessment of the study drug’s pharmacology
Moving to phase III trials without adequate assessment of the study drug’s pharmacology
 Side effects precluded reaching the therapeutic blood level shown to be effective in animals
Side effects precluded reaching the therapeutic blood level shown to be effective in animals
 Inadequately powered phase III trials to detect a modest but clinically meaningful treatment effect
Inadequately powered phase III trials to detect a modest but clinically meaningful treatment effect
Adapted from Gladstone DJ, Black SE, Hakim AM: Toward wisdom from failure: lessons from neuroprotective stroke trials and new directions. Stroke 33:2123–2136, 2002.58
A final consideration for future neuroprotection development programs would be to use agents that reduce infarct size when given early after AIS, but also enhance the natural recovery processes that occurs weeks and months later.61 This combination approach is appealing because both the reduction of infarct size engendered by the neuroprotective effect and the recovery-enhancing effect will contribute to improved stroke outcome. Current pessimism related to neuroprotection for AIS could be replaced by guarded optimism, but the organization and conduct of clinical trials will be more complex and difficult than in the past.
The most important future directions for AIS therapy are to extend the treatment window beyond the currently proven 4.5 hours from onset with IV tPA and to maximize the therapeutic benefit of this treatment within the currently documented time-to-treatment window. An attractive hypothesis for accomplishing both objectives is to use advanced imaging to identify AIS patients with extensive, persistent ischemic penumbra, the target of acute stroke therapy.3 Two imaging modalities, diffusion- or perfusion-weighted MRI (DWI/PWI; Fig. 31-2) and perfusion CT (Fig. 31-3) may be appropriate for this task.62,63 With DWI, regions of high-energy metabolism failure that developed cytotoxic edema can be readily identified and quantified as regions of hyperintensity that can be easily differentiated from normal brain regions. On PWI, various methods can be used to identify hypoperfused tissue, but approaches such as mean transit time mapping, time to peak mapping, and CBF mapping all have deficiencies in reliably differentiating among the ischemic core with very low CBF, the ischemic penumbra with moderate CBF, reductions, and oligemic tissue that is not destined for infarction.64,65 Currently, it is suggested that Tmax mapping that corrects for the arterial input of contrast in normal brain contralateral to the ischemic region likely provides the best distinction among these three ischemic regions. The ischemic penumbra is thought to be represented by regions that are abnormal on PWI by Tmax thresholding but are normal on DWI, the so-called DWI/PWI mismatch.66 Preliminary data support this concept because without therapy this DWI/PWI mismatch region is highly likely to demonstrate infarction on delayed T2 or fluid-attenuated inversion recovery (FLAIR) imaging. Magnetic resonance angiography (MRA) can also be acquired along with DWI and PWI and can depict the location of occlusion in large and medium-sized vessels.
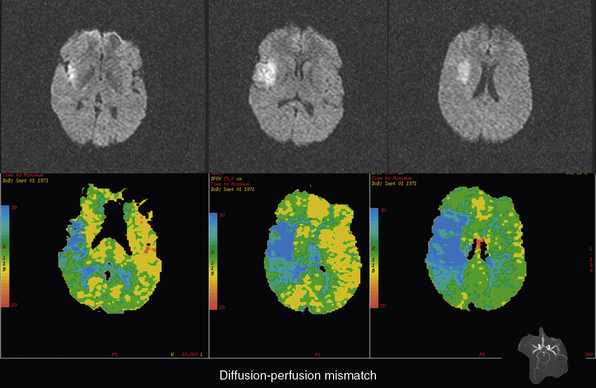
Figure 31-2 Example of a diffusion and perfusion mismatch on an acute magnetic resonance imaging (MRI) scan.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


















