Varied silent ischemic stroke (SS) prevalence occurs in patients with atrial fibrillation (AF). Stroke history is worth 2 points in the CHADS 2 scoring system. An unknown proportion of patients with AF with a CHADS 2 score of 0 or 1 have been undertreated for stroke prevention. We investigated SS risk factors using magnetic resonance imaging and estimated SS impact on clinical outcomes in patients with AF. We analyzed a total of 1,200 patients (400 with AF and 800 with sinus rhythm) who had brain magnetic resonance imaging performed for routine health checkups. Clinical outcomes including symptomatic stroke, dementia, and cognitive disorder were also evaluated in patients with AF (follow-up duration: 66.7 ± 35.9 months; range 10 to 162). SS was observed in 113 patients with AF (28.3%), which was significantly higher than that in 53 subjects (6.6%) with sinus rhythm (p <0.001, odds ratio [OR] 5.549). Independent risk factors for SS in patients with AF were age (OR 1.049), hypertension (OR 2.086), dyslipidemia (OR 2.073), and valvular AF (OR 3.157). Symptomatic stroke incidence during the follow-up was significantly greater in patients with AF with SS than without SS (5.6% vs 2.7% per year, respectively; p = 0.022, hazard ratio 1.787, 95% confidence interval 1.089 to 2.933). Using current scoring systems without correcting for subclinical stroke, clinicians have likely underestimated the stroke risk in low-risk patients with AF; thus many patients with AF might not receive optimal anticoagulation treatment. In conclusion, a screening tool for detecting SS could be considered for stroke risk evaluation in patients with AF, especially those with valvular AF, elderly patients, and patients with dyslipidemia or hypertension.
Atrial fibrillation (AF) is associated with an increased risk of cerebral infarction. However, it is sometimes overlooked that silent ischemic stroke (SS) is frequently seen in patients with asymptomatic AF. Therefore, stroke history is potentially underestimated in patients with AF. History of stroke or transient ischemic attack is valued at 2 points in the aforementioned scoring systems. Thus, an unknown, but likely significant, portion of patients with a CHADS 2 score of 0 or 1 are undertreated for stroke prevention. SS is defined as a cerebral infarction that is evident on brain imaging but is not associated with clinical symptoms. The SS prevalence in the general population ranges from 8% to 28%, with the differences mainly attributed to age. In the Framingham Study, SS occurs fairly frequently (10%) in subjects from a general population that was investigated for acute stroke symptoms. A population-based autopsy series revealed SS in 12.9% of the 966 subjects, and that diastolic blood pressure and AF appear to be strong predictors of silent cerebral infarction in the Japanese general population. In patients with AF, varied SS prevalence rates (13% to 48%) have been reported ( Table 1 ). In contrast, the SS incidence was approximately 3% per year among elderly people in 2 large population-based studies. Recent studies reported that SS presence can predict not only clinically overt future stroke but also dementia and decrease in cognitive function. Furthermore, the presence of silent infarct–like lesions was associated with depressive symptoms. Previous research reported that dementia might be related to AF even if no clinically overt stokes have occurred. Therefore, we can assume that silent infarctions may underlie the relation between dementia and AF. However, AF researchers have not yet strongly focused on the relation between AF and SS. Previous studies investigating AF and SS usually used computed tomography scanning as their SS detection tool, reporting lower SS frequencies than similar studies using magnetic resonance imaging (MRI). We used MRI to determine the prevalence of silent cerebral infarction in patients with AF without clinical neurologic symptoms and investigated the risk factors and prognostic impact of SS in patients with AF.
| Year | Authors | AF | NSR | Method | SS Prevalence |
|---|---|---|---|---|---|
| 1987 | Petersen et al | 29 | 29 | CT | 14:8 (48%:28%) |
| 1988 | Kempster et al | 54 | 168 | CT | 7:7 (13%:4%) |
| 1989 | Petersen et al | 30 | 30 | CT | 4:3 (13%:10%) |
| 1990 | Feinberg et al | 141 | — | CT | 36 (26%) |
| 1995 | Ezekowitz et al | 516 | CT | 76 (14.7%) | |
| 1995 | Hara et al | 72 | MRI | 32% | |
| 2012 | Kobayashi et al | 71 | 71 | MRI | 74.6%:57.7% |
Methods
Our study protocol was approved by the institutional review boards in Seoul National University Hospital and Healthcare System Gangnam Center and is in accordance with the Declaration of Helsinki. Patient consent was waived because it was not practicable to obtain consent from large numbers of patients for a retrospective review study, and the data were analyzed anonymously.
We enrolled 400 patients with AF (AF group) and 800 subjects with sinus rhythm (SR group) who had brain MRI performed from January 2000 to May 2012 at Seoul National University Hospital or Healthcare System Gangnam Center. All MRIs were performed during routine health checkups, and no patient had clinical neurologic symptoms or atypical symptoms such as dizziness or general weakness that were associated with a potential neurologic problem. If an expert neurologist decided that a patient’s symptom was possibly associated with old cerebral infarction on MRI, then the subject was excluded before case matching.
Patients in AF group were extracted from 1,959 patient records with electrocardiogram-documented AF. Patients with congenital structural heart disease were excluded. Among them, patients with follow-up medical data, who underwent brain MRI for the purpose of routine health checkup without symptoms, were 400. Patients in AF group were matched with 800 subjects in the SR group who were extracted from the records of 17,502 subjects with normal SR with routine health check brain MRI data. Any patients without detailed medical records or whose MRI data were not good for interpretation were also excluded before matching. The 2 groups (AF group and SR group) were matched with age, sex, and history of hypertension, dyslipidemia, and diabetes mellitus (DM). The detailed population data from which the sample groups were extracted and the data representing the Korean general population are described in Supplementary File 1 . The general population data in Korea were from the 2005 Korean National Health and Nutrition Examination Survey (KNHANES). The 2005 KNHANES was a cross-sectional study, which was a nationally representative survey on nutrition and health based on a comprehensive questionnaire in Korea.
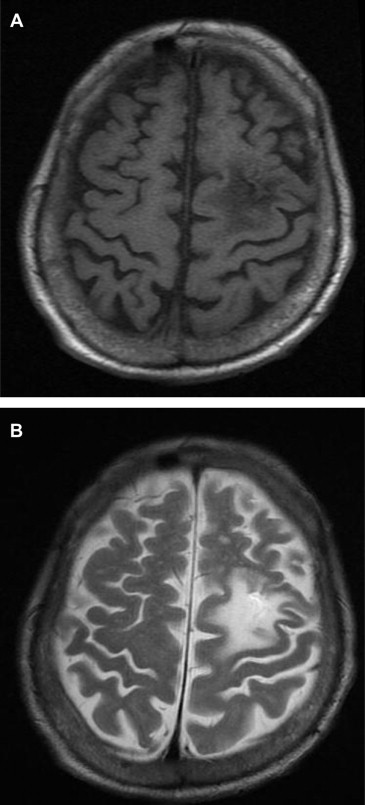
SS was defined as high-intensity areas identified on a T2-weighted image coinciding with low-intensity areas on a T1-weighted image on MRI ( Figure 1 ). MRI images were interpreted by more than 2 clinical neuroradiologists.
Uncorrected CHADS 2 or CHA 2 DS 2 -VASc scores are calculated irrespective of SS presence; for CHADS 2 risk index, 2 points are assigned for a history of stroke or transient ischemic attack and 1 point each for the presence of congestive heart failure (CHF), hypertension, age ≥75 years, and DM. In the CHA 2 DS 2 -VASc risk index, 2 points are assigned for stroke or transient ischemic attack history and age ≥75 years, and 1 point each is assigned for age 65 to 74 years, hypertension, DM, recent cardiac failure, vascular disease, and female gender. Corrected CHADS 2 or CHA 2 DS 2 -VASc scores regard SS as a history of stroke that is valued at 2 points.
Hypertension was defined as systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or current medication for hypertension treatment. DM was defined as a fasting blood sugar level of ≥126 mg/dl, a 2-hour blood sugar level of >200 mg/dl, or the use of medications to control blood sugar. Dyslipidemia was determined using the National Cholesterol Education Program Adult Treatment Panel III definition. Analyzed transthoracic echocardiographic data were obtained within 1 year before or after the date that MRI was performed. CHF was defined as a left ventricular ejection fraction of ≤35%. Valvular AF referred to cases with rheumatic mitral valve disease, prosthetic heart valve, or valve repair. Chronic kidney disease (CKD) was defined based on the presence of kidney damage or pathologically reduced glomerular filtration rate (Modification of Diet in Renal Disease–estimated glomerular filtration rate <60 ml/min/1.73 m 2 ) for >3 months, irrespective of the cause. Dementia and cognitive disorder were defined according to the neurologists’ diagnosis in clinical practice.
We compared the SS prevalence in the AF and SR groups, all of whom had undergone MRI for routine health checkups. In the AF group, SS risk factors considered in the multivariate analyses included age, sex, medical history (CKD, dyslipidemia, and other factors assessed by the CHADS 2 or CHA 2 DS 2 -VASc scoring system), and echocardiographic parameters. Clinical outcome data were analyzed including cerebral or cerebellar infarction, dementia, and cognitive disorder. Any patients that complained of, or were suspected to have, neurologic symptoms were referred to a neurologist for evaluation. The median follow-up period after MRI was 65.0 months (range 10 to 162).
To reduce the effect of selection bias and potential confounding factors in this observational study, we used propensity score matching with the R programing language to adjust for significant differences in baseline characteristics between the AF and SR groups. Comparison of characteristics among patients was performed using the chi-square test, with the Fisher’s exact test for categorical covariates or Student t test for continuous covariates. Multivariate analysis was performed by multiple logistic regression. All data are expressed as mean ± SD, if not otherwise specified. Outcome data were compared between subjects by a Cox regression model. p Values <0.05 were considered to indicate statistically significant differences. All analyses except for group matching were performed with SPSS, version 18.0 (SPSS Inc., Chicago, Illinois).
Results
The baseline characteristics of the enrolled 1,200 subjects (400 in the AF group and 800 in the SR group) are described in Table 2 . In the AF group, CKD and CHF prevalence was significantly greater than in the SR group. Patient characteristics of the AF group are described in Table 3 . Age, hypertension, dyslipidemia, CKD, left atrial size, medication status, and uncorrected CHADS 2 or CHA 2 DS 2 -VASc scores were significantly different between patients with versus those without SS.
| Variable | AF (n = 400) | SR (n = 800) | p Value |
|---|---|---|---|
| Age, yrs (range) | 66.60 ± 10.1 (26–90) | 65.83 ± 9.3 (26–88) | 0.228 |
| 20–39 | 3 (0.8%) | 6 (0.8%) | |
| 40–49 | 22 (5.5%) | 40 (5.0%) | |
| 50–59 | 70 (17.5%) | 139 (17.4%) | |
| 60–69 | 137 (34.3%) | 300 (37.5%) | |
| 70–79 | 134 (33.5%) | 277 (34.6%) | |
| >80 | 34 (8.5%) | 38 (4.8%) | |
| Women | 131 (32.8%) | 274 (34.3%) | 0.650 |
| Hypertension | 229 (57.3%) | 479 (59.9%) | 0.384 |
| DM | 95 (23.8%) | 184 (23.0%) | 0.772 |
| Dyslipidemia | 72 (18.0%) | 128 (16.0%) | 0.411 |
| CKD | 68 (17.0%) | 86 (10.8%) | 0.003 |
| CHF (ejection fraction ≤35%) | 28 (7.0%) | 1 (0.1%) | <0.001 |
| Variable | Total (n = 400) | SS Positive (n = 113) | SS Negative (n = 287) | p Value |
|---|---|---|---|---|
| Age (yrs) | 66.60 ± 10.1 | 69.82 ± 8.3 | 65.33 ± 10.5 | <0.001 |
| 25–39 | 3 (0.3%) | 0 | 3 (1.0%) | |
| 40–49 | 22 (6.0%) | 1 (0.9%) | 21 (7.3%) | |
| 50–59 | 70 (17.5%) | 10 (8.8%) | 60 (20.9%) | |
| 60–69 | 137 (34.3%) | 43 (38.1%) | 94 (32.8%) | |
| 70–79 | 134 (33.5%) | 47 (41.6%) | 87 (30.3%) | |
| 80– | 34 (8.5%) | 12 (10.6%) | 22 (7.7%) | |
| Women | 131 (32.8%) | 39 (34.5%) | 92 (32.1%) | 0.360 |
| Valvular AF | 26 (6.5%) | 11 (9.7%) | 15 (5.2%) | 0.081 |
| Underlying disease | ||||
| CHF (ejection fraction <35%) | 28 (7.0%) | 7 (6.2%) | 21 (7.3%) | 0.440 |
| Hypertension | 229 (57.3%) | 79 (69.9%) | 150 (52.3%) | 0.002 |
| DM | 95 (23.8%) | 29 (25.7%) | 66 (23.0%) | 0.329 |
| Vascular disease | 33 (8.3%) | 10 (8.8%) | 23 (8.0%) | 0.462 |
| CKD | 68 (17.0%) | 26 (23.0%) | 42 (14.6%) | 0.034 |
| Dyslipidemia | 72 (18.0%) | 29 (25.7%) | 43 (15.0%) | 0.010 |
| Echocardiography | ||||
| Left ventricular ejection fraction (%) | 58.55 ± 8.8 | 58.75 ± 8.6 | 58.46 ± 8.9 | 0.779 |
| Left atrial size (mm) | 47 ± 8.7 | 50 ± 10.3 | 46 ± 7.4 | 0.014 |
| Medication | ||||
| None | 173 (43.3%) | 38 (33.6%) | 135 (47.0%) | |
| Aspirin | 124 (31.0%) | 35 (31.0%) | 89 (31.0%) | |
| Warfarin | 103 (25.8%) | 40 (35.4%) | 63 (22.0%) | |
| Uncorrected | 1.34 ± 0.84 | 1.05 ± 0.89 | 0.003 | |
| CHADS 2 score | ||||
| 0 | 95 (26.0%) | 15 (14.7%) | 80 (29.4%) | |
| 1 | 164 (43.9%) | 46 (45.1%) | 118 (43.4%) | |
| 2 | 89 (23.8%) | 32 (31.4%) | 57 (21.0%) | |
| 3 | 24 (6.4%) | 9 (8.8%) | 15 (5.5%) | |
| 4 | 2 (0.5%) | 0 (0.0%) | 2 (0.7%) | |
| Uncorrected | 2.50 ± 1.15 | 2.03 ± 1.33 | 0.001 | |
| CHA 2 DS 2 -VASc score | ||||
| 0 | 38 (10.2%) | 1 (1.0%) | 37 (13.6%) | |
| 1 | 88 (23.5%) | 21 (20.6%) | 67 (24.6%) | |
| 2 | 99 (26.5%) | 31 (30.4%) | 68 (25.0%) | |
| 3 | 91 (24.3%) | 29 (28.4%) | 62 (22.8%) | |
| 4 | 43 (11.5%) | 15 (14.7%) | 28 (10.3%) | |
| 5 | 15 (4.0%) | 5 (4.9%) | 10 (3.7%) | |
| Follow-up duration, months (range) | 66.7 ± 35.9 (10–162) | 65.9 ± 34.8 (10–157) | 66.9 ± 36.4 (12–162) | 0.779 |
SS lesions were found in 113 (28.3%) of 400 patients in the AF group, which was a significantly greater prevalence than in the SR group (53 [6.6%] of 800). There were 29 (7.3%) of 400 patients with lacunar SS lesions and 90 (22.5%) of 400 with nonlacunar SS lesions in the AF group. There were 28 (3.5%) of 800 patients with lacunar SS lesions and 26 (3.3%) of 800 with nonlacunar SS lesions in the SR group. This was a consistent finding in prevalence analyses performed separately in each gender group (men: 74 [27.5%] of 269 in the AF group vs 34 [6.5%] of 526 in the SR group, women: 39 [29.8%] of 131 in the AF group vs 19 [6.9%] of 274 in the SR group). Of the 26 patients with valvular AF, 11 patients (42.3%) had SS, which is significantly higher than patients with nonvalvular AF (27.3%, p <0.001).
Follow-up duration for patients with AF was 66.7 ± 35.9 months (range 10 to 162, median 65.0). Clinically overt stroke events totaled 80 (3.6% per year) during the follow-up period (65 cerebral, 9 cerebellar, and 6 lacunar infarctions). Annual stroke risk was elevated according to increased uncorrected CHADS 2 or CHA 2 DS 2 -VASc scores. Overt stroke incidence was significantly greater in patients with AF with SS (5.6% per year) than those without SS (2.7% per year) when age, gender, CHF, hypertension, DM, dyslipidemia, vascular disease, and CKD were adjusted for (p = 0.022, hazard ratio 1.787, 95% confidence interval [CI] 1.089 to 2.933; Figure 2 ). However dementia or cognitive disorder incidences were not associated with SS prevalence in our study (3.3% vs 1.9% per year, p = 0.269; Figure 2 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


