A localized hypertrophy of the subaortic segment of the ventricular septum—ventricular septal bulge (VSB)—has been frequently described in series of elderly population, but its prevalence with age, clinical correlates, and impact on cardiac function and exercise capacity remain uncertain. We explored these associations in a cross-sectional sample without known cardiac disease from the Baltimore Longitudinal Study of Aging. We randomly selected 700 participants (50% men, mean age 64 ± 15, range 26 to 95 years) and reviewed their echocardiograms. We identified 28 men and 21 women with VSB (7% overall prevalence). The prevalence of VSB significantly increased with age in both genders (p <0.0001). In multivariate logistic regression including hypertension and other cardiovascular risk factors, only age displayed a significant independent association with VSB (OR 1.06 per year, 95% confidence interval 1.03 to 1.10, p = 0.0001). After multiple adjustments, participants with VSB compared with those without had enhanced global left ventricular contractility (fractional shortening 41 ± 1.3 vs 38 ± 0.3%, p = 0.04; ejection fraction 71 ± 1.6 vs 67 ± 0.4%, p = 0.06; systolic velocity of the mitral annulus 8.4 ± 0.1 vs 8.9 ± 0.3, p = 0.06), and larger aortic root diameters (3.3 ± 0.06 vs 3.1 ± 0.02 cm, p = 0.02). In subgroup of participants who completed a maximal treadmill test (177 women and 196 men), those with VSB (19, 5.1%) had significantly lower peak oxygen consumption than their counterparts (19.6 ± 3.8 vs 22.9 ± 6.6 ml/kg/min, p = 0.03). However, this association was no longer significant after multiple adjustments. In conclusion, the presence of VSB is independently associated with older age and determines enhanced left ventricular contractility, without any evident impact on exercise capacity.
A localized hypertrophy of the subaortic segment of the ventricular septum has been frequently described in elderly population and variously termed subaortic ventricular septal bulge (VSB), sigmoid-shaped septum, and localized or discrete upper septal hypertrophy. It has been considered mainly as an incidental finding associated with older age and hypertension, although some authors have suggested that it may be part of the spectrum of hypertrophic cardiomyopathy and may determine some degree of left ventricular (LV) obstruction, which in turn may potentially cause symptoms such as syncope and dyspnea on effort.
However, the prevalence with age and clinical correlates of this particular feature of the LV septum has been little explored in populations free from cardiac disease, as well as its potential impact on LV function and structure after accounting for all potential age-related co-morbidities. Furthermore, because of few previous reports showing increased LV outflow tract (LVOT) gradients with VSB under stress conditions, as it is generally described in patients with labile hypertrophic cardiomyopathy, it is reasonable to hypothesize that the VSB may impair peak exercise capacity. We, therefore, assessed the prevalence of VSB in a large sample of healthy-aging women and men dispersed over a wide age range enrolled in the Baltimore Longitudinal Study of Aging (BLSA), explored clinical correlates beyond age and hypertension, and examined the potential impact of VSB on maximal exercise capacity.
Methods
The study sample was drawn from a population of healthy adults participating in the BLSA, an ongoing prospective study of normative aging. Participants are enrolled in the study if they are healthy at baseline and undergo 3 days of medical examinations approximately every 2 years. Seven hundred BLSA participants without a history of coronary artery disease and/or heart failure and without valvular disease greater than mild in severity and/or LV systolic dysfunction (defined as LV ejection fraction <40%) at the index visit were randomly selected from the BLSA echocardiography database, to cover a wide age distribution, according to the first aim of the study. An equal number of women and men was selected, with comparable age distribution (men: mean age = 64 ± 15, range 27 to 94 years; women: mean age = 63 ± 15, range 26 to 95 years; p = 0.20). The study protocol was approved by the National Institute on Aging and the MedStar Health Research Institute (Baltimore, Maryland). All participants provided informed participation consent.
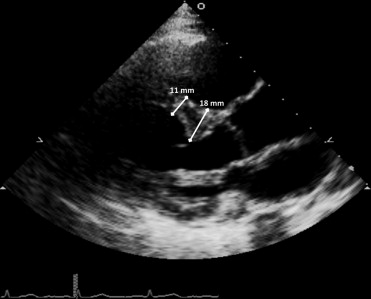
All transthoracic echocardiograms were performed at rest with the same echocardiographic instrument (HP Sonos-5500; Philips, Andover, Massachusetts). Echocardiographic images were retrieved from the BLSA digital archive (started in 2004) and reviewed independently by 1 trained reader (OM) and 1 experienced echocardiographer (MD). Measurements to characterize the presence of a subaortic VSB were made in all 700 selected participants from the 2-dimensional parasternal long-axis view at end-diastole. According to a combination of criteria applied in previous studies, the presence of subaortic VSB was defined as a proximal focal area (within the first third of total septal length) of localized septal hypertrophy with a dune-like structure protruding in the LVOT, a thickness ≥13 mm in men and ≥12 mm in women, and more than 50% greater than the thickness of the septum at its mid-distal point ( Figure 1 ). Systolic anterior movement of the mitral valve was evaluated in the same image but never observed. The aortic root and the LVOT diameters were measured from the same view. LV posterior wall thickness, dimensions, mass, volumes, ejection fraction, and fractional shortening were calculated as previously reported. Left atrial volume was measured by planimetry in the apical 4-chamber view. The latter, the LV mass and volumes were indexed to body surface area. Prevalence of LV concentric remodeling and hypertrophy (combining concentric and eccentric) were estimated using gender-specific cutoffs for LV mass index. Tissue Doppler diastolic (Em) and systolic (Sm) velocities of the septal and lateral mitral annulus were also measured and averaged. The ratio between the mitral flow E- and A-wave velocities (E/A ratio) was considered as an index of LV relaxation, whereas the ratio between E and mean Em (E/Em ratio) as an index of LV filling pressures. LVOT velocities and velocity-time integral were studied at rest by pulse-wave Doppler from the apical window. Three cardiac cycles were averaged for each one of these functional measurements.
Hypertension was defined as mean systolic blood pressure ≥140 mm Hg and/or mean diastolic blood pressure ≥90 mm Hg on 3 consecutive measurements at the brachial artery right before the echocardiography or as use of antihypertensive medications. Diabetes mellitus was diagnosed according to the American Diabetes Association criteria or use of diabetes medications. Participants were classified as physically active if reporting ≥1,000 kcal/week of exercise activity at medical interview. Waist circumference was defined as the minimal abdominal circumference between the lower edge of the rib cage and the iliac crests, and it was measured with a flexible tape while maintaining close contact with skin, without compressing the underlying tissues and with participants in a standing position and breathing normally. Body mass index was calculated as weight divided by height-squared (kg/m 2 ). Participants were identified obese if their body mass index was ≥30 kg/m 2 . The glomerular filtration rate, calculated by the simplified modification of diet in renal disease formula, was used to determine renal function, and renal failure was defined as glomerular filtration rate <60 ml/min/1.73 m 2 at the index visit. Fasting, 2-hour postchallenge plasma glucose, plasma triglyceride, and total cholesterol concentrations were measured as previously reported.
Oxygen consumption was measured continuously during a modified Balke protocol, and the highest value was termed peak VO 2 , expressed in milliliters per kilogram per minute, as previously reported. Participants with a respiratory exchange ratio <1.1, a marker of a maximal treadmill effort, were excluded from the analysis. We also estimated the change per year in peak VO 2 for those participants who had 1 or more additional peak VO 2 evaluation available after the index visit.
Data were analyzed using the SAS package (version 9.3; SAS Institute Inc., Cary, North Carolina) and are presented as percentages and means ± SD (or SE of the adjusted means). A Student t test or chi-square test was used as appropriate to assess statistical difference between participants with and without VSB. Adjusted means of echocardiographic measurements were compared between the 2 groups by analysis of covariance before and after controlling for significant potential confounders. Univariate and multivariate logistic regressions were used to examine the association of clinical variables selected from previous significant comparisons with the presence of VSB. Univariate and multivariate linear regressions were used to evaluate the impact of the presence of VSB on exercise capacity estimated by peak VO 2 (or the change in peak VO 2 per year), accounting for variables already known to have a significant impact on oxygen consumption. Collinearity was assessed calculating the variance inflation factor that was found acceptable (<1.5) in all models. Statistical significance was set at p <0.05.
Results
According to our diagnostic criteria, participants with VSB (28 men and 21 women, 7% overall prevalence) had higher proximal septum thickness and proximal to midseptum thickness ratio than those without VSB, whereas there was no difference in midseptum thickness ( Table 1 ). When comparing men and women within a group, women had significantly smaller wall thicknesses than men, except for proximal septum thickness in those with VSB ( Table 1 ).
| Variable | Ventricular Septal Bulge | p Value | |
|---|---|---|---|
| No (n = 651) | Yes (n = 49) | ||
| Proximal septum thickness (overall, men/women, cm) | 1.06 1.15/0.97 † | 1.64 1.7/1.6 | <0.0001 ∗ |
| Mid septum thickness (overall, men/women, cm) | 0.91 0.99/0.83 † | 0.93 1/0.84 † | >0.46 ∗ |
| Ratio proximal to mid (overall, men/women) | 1.20 1.19/1.21 | 1.80 1.69/1.95 † | <0.0001 ∗ |
| Age (years) | 65 ± 14 | 76 ± 11 | <0.0001 |
| Men | 50% | 57% | 0.30 |
| Heart rate (beats/min) | 67 ± 10 | 70 ± 13 | 0.10 |
| Systolic blood pressure (mm Hg) | 117 ± 15 | 122 ± 18 | 0.02 |
| Diastolic blood pressure (mm Hg) | 66 ± 9 | 65 ± 10 | 0.26 |
| Hypertension | 37% | 55% | 0.01 |
| On antihypertensive medications | 35% | 51% | 0.02 |
| Body mass index (kg/m 2 ) | 27 ± 5 | 27 ± 4 | 0.62 |
| Waist circumference (cm) | 90 ± 13 | 91 ± 14 | 0.42 |
| Obesity | 23% | 20% | 0.63 |
| Fasting plasma glucose (mg/dL) | 91 ± 18 | 97 ± 21 | 0.01 |
| 2-h Postchallenge plasma glucose (mg/dL) | 124 ± 52 | 137 ± 54 | 0.11 |
| Diabetes mellitus | 10% | 20% | 0.03 |
| Glomerular filtration rate (ml/min/1.73 m 2 ) | 78 ± 18 | 70 ± 20 | 0.003 |
| Renal failure | 15% | 25% | 0.06 |
| Triglyceride (mg/dl) | 103 ± 62 | 119 ± 64 | 0.10 |
| Total cholesterol (mg/dl) | 192 ± 36 | 192 ± 37 | 0.89 |
∗ p Value also for comparison of participants with and without ventricular septal bulge of the same gender.
† p Value for comparison between men and women within the group <0.003.
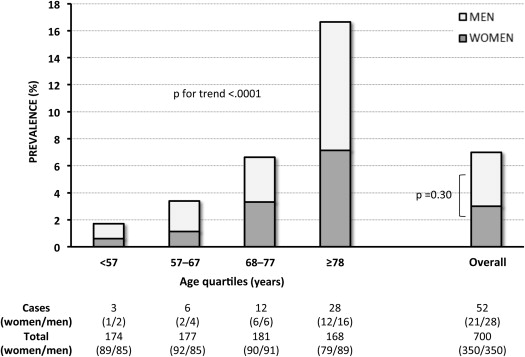
Clinical characteristics of the study population by the presence of VSB are listed in Table 1 . The prevalence of VSB significantly increased with age in both genders ( Figure 2 ), from 1.7% in those younger than 57 years to 16.7% in those older than 78 years. There was no significant difference in the prevalence of VSB between gender in each age quartile and in the overall population ( Figure 2 ). Nineteen of the 49 subjects with VSB (mean age 73 years, range 60 to 87) underwent 1 or more echocardiography examinations (total 63, mean per subject 3, range 2 to 6) before the index visit (mean time before index visit 5.1 years, range 2 to 7). Eleven of them (58%) had 1 or more previous echocardiograms meeting criteria for the diagnosis of VSB, whereas the remaining 8 subjects did not meet these criteria before the index visit.
Significant determinants of VSB in univariate analysis are listed in Table 2 . In multivariate logistic regression, the presence of VSB was significantly associated only with older age, and this result remained substantially unchanged after performing stepwise backward elimination ( Table 2 ). No significant difference was found if waist circumference or obesity was substituted for body mass index, renal failure for glomerular filtration rate, or use of antihypertensive medications for hypertension (data not shown).
| Univariate Analysis | Multivariate Analysis | Reduced Analysis | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age (year) | 1.07 (1.04–1.10) | <0.0001 | 1.06 (1.03–1.10) | 0.0001 | 1.07 (1.04–1.10) | <.0001 |
| Male gender | 1.36 (0.76–2.45) | 0.30 | 1.33 (0.71–2.51) | 0.38 | ||
| Hypertension | 2.07 (1.16–3.72) | 0.015 | 1.02 (0.51–2.05) | 0.95 | ||
| Systolic blood pressure (mm Hg) | 1.02 (1.00–1.04) | 0.016 | 1.01 (0.99–1.03) | 0.37 | ||
| Glomerular filtration rate (ml/min/1.73 m 2 ) | 0.98 (0.96–0.99) | 0.003 | 0.99 (0.97–1.01) | 0.23 | ||
| Body mass index (kg/m 2 ) | 1.02 (0.96–1.08) | 0.62 | 1.03 (0.96–1.11) | 0.39 | ||
| Diabetes mellitus | 2.24 (1.07–4.68) | 0.03 | 1.10 (0.42–2.87) | 0.85 | ||
| Fasting plasma glucose (mg/dl) | 1.01 (1.00–1.02) | 0.02 | 1.01 (0.99–1.03) | 0.28 | ||
Table 3 lists the difference in echocardiographic structural and functional parameters by the presence of VSB after accounting for confounders. Participants with VSB were found to have increased systolic function parameters (such as fractional shortening, LV ejection fraction, and systolic velocity of the mitral annulus), and they also had larger aortic root diameters compared with those without VSB, even after adjusting for body surface area.
| Variable | Ventricular Septal Bulge | p Value | p Value (Unadjusted) | |
|---|---|---|---|---|
| No (n = 651) | Yes (n = 49) | |||
| E/A ratio ∗ | 1.04 ± 0.01 | 0.97 ± 0.05 | 0.23 | <0.001 |
| Em (cm/s) ∗ | 9.4 ± 0.1 | 9.3 ± 0.3 | 0.71 | <0.0001 |
| E/Em ratio ∗ | 8.3 ± 0.1 | 8.6 ± 0.4 | 0.59 | 0.007 |
| Left atrial volume index (mL/m 2 ) | 16.3 ± 0.3 | 15.7 ± 1.1 | 0.61 | 0.40 |
| LVOT velocity at rest (m/sec) ∗ | 1.11 ± 0.01 | 1.13 ± 0.03 | 0.53 | 0.46 |
| LVOT velocity time integral at rest ∗ | 23.7 ± 0.19 | 24.3 ± 0.73 | 0.39 | 0.22 |
| LVOT diameter (cm) † | 2.05 ± 0.01 | 2.09 ± 0.03 | 0.19 | 0.55 |
| Aortic root diameter (cm) † | 3.1 ± 0.02 | 3.3 ± 0.06 | 0.02 | 0.003 |
| LV posterior wall (cm) † | 0.95 ± 0.01 | 0.94 ± 0.02 | 0.80 | 0.06 |
| LV mass index (g/m 2 ) | 74.3 ± 0.84 | 73.4 ± 3.28 | 0.79 | 0.27 |
| LV remodeling categories | ||||
| No LV remodeling | 43% | 45% | 0.78 | 0.06 |
| Concentric LV remodeling | 46% | 48% | 0.77 | 0.06 |
| LV hypertrophy | 11% | 7% | 0.40 | 0.96 |
| End-diastolic volume index (mL/m 2 ) | 40.7 ± 0.6 | 41.8 ± 2.2 | 0.63 | 0.87 |
| End-systolic volume index (mL/m 2 ) | 14.4 ± 0.3 | 15.6 ± 1.1 | 0.28 | 0.72 |
| LV ejection fraction (%) ∗ | 67 ± 0.4 | 71 ± 1.6 | 0.06 | 0.004 |
| Fractional shortening (%) ∗ | 38 ± 0.3 | 41 ± 1.3 | 0.04 | 0.003 |
| Sm (cm/s) ∗ | 8.4 ± 0.1 | 8.9 ± 0.3 | 0.06 | 0.97 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


