Co-morbidities have typically been considered as prevalent cardiovascular risk factors and cardiovascular diseases rather than systematic measures of general co-morbidity burden in patients who underwent percutaneous coronary intervention (PCI). Charlson co-morbidity index (CCI) is a measure of co-morbidity burden providing a means of quantifying the prognostic impact of 22 co-morbid conditions on the basis of their number and prognostic impact. The study evaluated the impact of the CCI on cardiac mortality and major adverse cardiovascular events (MACE) after PCI through analysis of the Nobori-2 study. The prognostic impact of CCI was studied in 3,067 patients who underwent PCI in 4,479 lesions across 125 centers worldwide on 30-day and 1- and 5-year cardiac mortality and MACE. Data were adjusted for potential confounders using stepwise logistic regression; 2,280 of 3,067 patients (74.4%) had ≥1 co-morbid conditions. CCI (per unit increase) was independently associated with an increase in both cardiac death (odds ratio [OR] 1.47 95% confidence interval [CI] 1.20 to 1.80, p = 0.0002) and MACE (OR 1.29 95% CI 1.14 to 1.47, p ≤0.0011) at 30 days, with similar observations recorded at 1 and 5 years. CCI score ≥2 was independently associated with increased 30-day cardiac death (OR 4.25, 95% CI 1.24 to 14.56, p = 0.02) at 1 month, and this increased risk was also observed at 1 and 5 years. In conclusion, co-morbid burden, as measured using CCI, is an independent predictor of adverse outcomes in the short, medium, and long term. Co-morbidity should be considered in the decision-making process when counseling patients regarding the periprocedural risks associated with PCI, in conjunction with traditional risk factors.
The average age of patients who underwent percutaneous coronary intervention (PCI) has increased over time in western populations in part through an increased prevalence of cardiovascular diseases in the increasingly elderly population. This aging population who underwent PCI has a greater burden of co-morbid conditions that are known to have an important prognostic impact on patients with a variety of cardiovascular diseases. In patients who underwent PCI, co-morbidities have typically been considered as prevalent cardiovascular risk factors and other cardiovascular diseases rather than systematic measures of general co-morbidity burden. The Charlson co-morbidity index (CCI) is a measure of co-morbidity burden and provides a means of quantifying the prognostic impact of 22 co-morbid conditions on the basis of their number and their individual prognostic impact by means of a score that was developed as a prognostic indicator for patients with a variety of medical conditions. The CCI has been shown to independently predict adverse mortality outcomes in a number of cardiovascular diseases. Analysis of the prevalence and prognostic impact of co-morbidities as defined by the CCI is less clearly defined in patients who underwent PCI. Previous studies have reported that the CCI is an important predictor of mortality, mortality and myocardial infarction (MI), and major adverse cardiovascular and cerebrovascular events although have not systematically looked at prevalence and type of co-morbidities or the prognostic impact of CCI on short- and long-term outcomes or on end points, such as target-vessel revascularization, major bleeding, or stent thrombosis. We have, therefore, evaluated the prevalence of co-morbid conditions that make up the CCI and its impact on cardiac mortality, major adverse cardiovascular events (MACE), stent thrombosis, and major bleeding complications in an all-comer population through analysis of data derived from a large, prospective, multicenter study conducted in 125 centers across Europe and Asia using only drug-eluting stents (DES)—the Nobori-2 study.
Methods
The Nobori biolimus-eluting stent (Nobori BES; Terumo, Tokyo, Japan) is an abluminally coated DES with a matrix containing the high lipophilic, sirolimus-analogue, biolimus A9 drug, and the biodegradable poly-lactic acid polymer. Nobori-2 is a prospective, multicenter study conducted in 125 centers across Europe and Asia to investigate the performance of the Nobori DES system in an all-comers clinical setting with the only exclusion criterion used being the patient’s refusal or inability to provide written informed consent. All patients who had at least 1 Nobori DES implanted or attempted were included in the analysis. All patients signed an informed consent form reviewed and approved by the Institutional Review Board or Ethics Committee of each participating centers.
Co-morbidity was assessed using the CCI without age weighting. The CCI is a scoring system that involves weighting co-morbid conditions on the basis of their number and severity and was developed as a prognostic indicator for patients with a variety of medical conditions. The co-morbidities defined by Charlson et al use a point system with each weighted by a value depending on the prognostic impact of co-morbid condition ranging from 1 to 6 points ( Table 1 ). The scores are summated to calculate a CCI; therefore, patients with no co-morbidities had a CCI of 0, those with only 1 co-morbidity weighted as 1 had a CCI of 1, patients with 2 co-morbidities where both were weighted 1 or 1 co-morbidity was weighted 2 had a CCI of 2, and the patients where the sum of the weighted points of co-morbidities was ≥3 had a CCI ≥3. The CCI was collected at baseline, and outcomes were stratified by CCI score: CCI 0, 1, 2, and ≥3.
| Variable | Points |
|---|---|
| Myocardial infarction | 1 |
| Congestive heart failure | 1 |
| Peripheral vascular disease | 1 |
| Cerebrovascular disease | 1 |
| Dementia | 1 |
| Chronic obstructive pulmonary disease | 1 |
| Connective tissue disease | 1 |
| Peptic ulcer disease | 1 |
| Diabetes mellitus | 1 if uncomplicated 2 if end-organ damage |
| Moderate to severe chronic kidney disease | 2 |
| Hemiplegia | 2 |
| Leukemia | 2 |
| Malignant lymphoma | 2 |
| Solid tumour | 2 6 if metastatic |
| Liver disease | 1 if mild 3 if moderate to severe |
| AIDS | 6 |
An acute coronary syndrome (ACS) was defined as typical symptoms with ischemic electrocardiographic changes including ST-segment elevation and non–ST-segment elevation and/or laboratory evidence of myocardial damage. MACE was defined as a composite of cardiac death, MI, and target-vessel revascularization. Major bleeding complications were defined using the Safety and efficacy of enoxaparin in percutaneous coronary intervention patients, an international randomized evaluation (STEEPLE) major bleeding criterion. Stent thrombosis was a composite of definite and probable acute, subacute, late, or very late stent thrombosis as defined by the Academic Research Consortium.
All clinical, demographic, and outcome data were collected into a Web-based data management system co-ordinated and analyzed by independent companies (KIKA Medical, Paris, France, and SBD Analytics, Bekkevoort, Belgium, respectively). Clinical follow-up data included the documentation of adverse events, death, MI, repeat revascularization, stent thrombosis, bleeding, and angina status.
Follow-up was performed at 1, 6, and 12 months and yearly up to 5 years. An independent clinical events committee adjudicated all clinical end points.
Categorical variables are presented as numbers and percentages and were compared with the Fisher’s exact test. Continuous variables are expressed as mean ± SD and are compared between CCI groups using the Wilcoxon rank-sum test. All analyses were performed according to the intention-to-treat principle. Univariate odds ratios and 95% CI were calculated using a univariate logistic regression model. For adjusted odds ratios, multivariate logistic regression models were constructed with a stepwise selection method: beginning with an intercept-only model, variables were added repetitively if their p value was 0.25, with the most significant variable being added to the model. At each step, the model was run, and variables with a p value >0.05 were again deleted. This process was repeated until no more variables with a p <0.25 were found to be added or could be dropped if p >0.05. Covariates entered into the model included age, gender, Charlson score, syndrome of presentation, diabetes, renal function, bifurcation lesion, ACC lesion classification, lesion calcification, multivessel disease, and lesion length. All p values were 2 sided, and a p value <0.05 was regarded as statistically significant. SAS, version 8.02 (The SAS Institute, Cary, North Carolina) was used for analysis.
Results
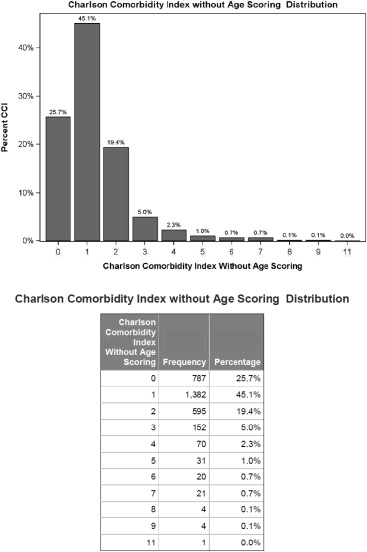
The Nobori-2 study enrolled 3,067 patients from 125 centers across the world who underwent PCI to 4,479 lesions and were followed up prospectively for 5 years. Follow-up data were available in 100% at 1 month, 99.9% at 1 year (1 patient withdrew consent), and 89.3% of patients at 5 years (3 patients withdrew consent and 326 patients were lost to follow-up). The mean age (±SD) of patients was 64.4 ± 11.0 years, 2,391 of 3,067 (78%) were men, and 1,640 of 3,067 (53.6%) underwent PCI for ACS indications. The CCI was collected at baseline, and mean (±SD) CCI for the whole cohort was 1.24 ± 1.24. Figure 1 illustrates the frequency distribution of CCI among the cohort: 787 of 3,067 patients (25.6%) had no co-morbid conditions, while the most prevalent CCI score was 1 (1,382 of 3,067, 45.1%).
The cohort was stratified by CCI score: CCI 0, 1, 2, and ≥3. Baseline clinical characteristics of participants by CCI group and for the whole cohort are presented in Table 2 . The mean age increased across increasing CCI groups: 63.7 ± 10.8 year old in the CCI 0 group and 69.5 ± 10.3 in the CCI ≥3 group (p <0.0001). The prevalence of cardiovascular risk factors and co-morbid conditions also increased across the CCI groups. The most common co-morbidities found in the CCI were previous acute myocardial infarction (AMI, 987 of 2,977, 33.2%) and diabetes (888 of 3,013, 29.5%), whereas the most prevalent noncardiovascular co-morbidities included chronic obstructive pulmonary disease (COPD) (121 of 2,916, 4.2%), cancer (solid tumor) 75 of 2,912 (2.6%), and peptic ulcer disease (81 of 2,917, 2.8%). Table 3 illustrates procedural and lesion characteristics by CCI group. As CCI score increased, the proportion of patients with 3-vessel disease increased, and lesions were more complex as evidenced by increasing calcification, tortuosity, and increased prevalence of type C lesions.
| Variable | CCI 0 (n=787) | CCI 1 (n=1382) | CCI 2 (n=595) | CCI≥3 (n=303) | Whole cohort (n=3,067) | P-Value |
|---|---|---|---|---|---|---|
| Age (years) | 63.7±10.8 | 63.0±11.0 | 65.8±10.5 | 69.5±10.3 | 64.4±11.0 | <0.0001 |
| Men | 629 (79.9%) | 1,085 (78.5%) | 455 (76.5%) | 222 (73.3%) | 2,391 (78.0%) | 0.0853 |
| Previous percutaneous coronary intervention | 186 (24.1%) | 454 (33.4%) | 205 (34.8%) | 128 (42.5%) | 973 (32.2%) | <0.0001 |
| Current smoker | 157 (22.3%) | 364 (29.1%) | 133 (25.1%) | 53 (19.0%) | 707 (25.6%) | 0.0003 |
| Ex-smoker | 235 (33.3%) | 427 (34.1%) | 192 (36.3%) | 103 (36.9%) | 957 (34.6%) | 0.575 |
| Hypercholesterolemia | 513 (68.1%) | 933 (70.6%) | 428 (74.4%) | 218 (73.9%) | 2,092 (71.1%) | 0.0554 |
| Hypertension | 507 (66.4%) | 867 (63.8%) | 451 (76.7%) | 255 (85.6%) | 2,080 (69.1%) | <0.0001 |
| Family history of heart disease | 262 (39.2%) | 438 (38.1%) | 153 (31.9%) | 65 (28.3%) | 918 (36.3%) | 0.0021 |
| Acute coronary syndrome presentation | 211 (26.8%) | 863 (62.5%) | 382 (64.2%) | 184 (60.7%) | 1,640 (53.6%) | <0.0001 |
| Charlson comorbidity component (%) | ||||||
| Previous acute myocardial infarction | 0 (0%) | 588 (43.4%) | 261 (44.9%) | 138 (46.9%) | 987 (33.2%) | <0.0001 |
| Heart failure | 17 (2.3%) | 28 (2.2%) | 30 (5.3%) | 39 (13.2%) | 114 (3.9%) | <0.0001 |
| Peripheral vascular disease | 0 (0%) | 24 (1.8%) | 82 (14.4%) | 86 (29.2%) | 192 (6.6%) | <0.0001 |
| Previous stroke | 0 (0%) | 18 (1.4%) | 42 (7.4%) | 68 (23.1%) | 128 (4.4%) | <0.0001 |
| Dementia | 0 (0%) | 0 (0%) | 2 (0.35%) | 6 (2.0%) | 8 (0.3%) | <0.0001 |
| Chronic obstructive pulmonary disease | 0 (0%) | 16 (1.2%) | 46 (8.1%) | 59 (19.7%) | 121 (4.2%) | <0.0001 |
| Connective tissue disease | 0 (0%) | 4 (0.3%) | 3 (0.5%) | 5 (1.7%) | 12 (0.4%) | 0.0031 |
| Peptic ulcer | 0 (0%) | 15 (1.1%) | 31 (5.5%) | 35 (11.9%) | 81 (2.8%) | <0.0001 |
| Mild liver disease | 0 (0%) | 4 (0.3%) | 6 (1.1%) | 9 (3.1%) | 19 (0.7%) | <0.0001 |
| Moderate-severe liver disease | 0 (0%) | 0 (0%) | 0 (0%) | 4 (1.4%) | 4 (0.1%) | <0.0001 |
| Hemiplegia | 0 (0%) | 0 (0%) | 0 (0%) | 10 (3.4%) | 10 (0.3%) | 0.0001 |
| Moderate-severe chronic kidney disease | 0 (0%) | 0 (0%) | 14 (2.5%) | 89 (29.7%) | 103 (3.5%) | <0.0001 |
| Diabetes | 0 (0%) | 262 (19.4%) | 422 (71.2%) | 204 (67.6%) | 888 (29.5%) | <0.0001 |
| Diabetes (end organ) | 0 (0%) | 0 (0%) | 0 (0%) | 87 (29.6%) | 87 (3.0%) | <0.0001 |
| Leukemia | 0 (0%) | 0 (0%) | 1 (0.2%) | 2 (0.7%) | 3 (0.1%) | 0.0071 |
| Lymphoma | 0 (0%) | 0 (0%) | 3 (0.5%) | 3 (1.0%) | 6 (0.2%) | 0.0004 |
| Solid tumor | 0 (0%) | 0 (0%) | 19 (3.4%) | 56 (18.9%) | 75 (2.6%) | <0.0001 |
| Solid tumor + metastasis | 0 (0%) | 0 (0%) | 0 (0%) | 3 (1%) | 3 (0.1%) | 0.001 |
| AIDS | 0 (0%) | 0 (0%) | 0 (0%) | 2 (0.7%) | 2 (0.1%) | 0.0101 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


